BISPHENOL A DIGLYCIDYL ETHER RESIN
Synonym(s):2,2-Bis[4-(glycidyloxy)phenyl]propane;4,4′-Isopropylidenediphenol diglycidyl ether;BADGE;Bisphenol A diglycidyl ether;Epoxy-Resin
- CAS NO.:1675-54-3
- Empirical Formula: C21H24O4
- Molecular Weight: 340.41
- MDL number: MFCD00080480
- EINECS: 216-823-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-04-10 20:44:46
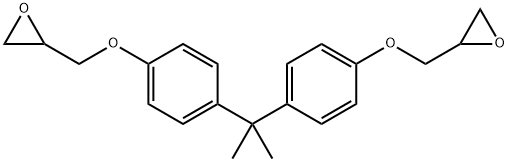
What is BISPHENOL A DIGLYCIDYL ETHER RESIN?
Description
Most epoxy resins result from polymerization of bisphenol A diglycidyl ether (BADGE). Delayed hypersensitivity is caused by the low-molecular-weight monomer BADGE (MW 340 g/mol), the dimer having a much lower sensitization power. This allergen caused contact dermatitis in six workers in a plant producing printed circuits boards made of copper sheets and fiberglass fabric impregnated with a brominated epoxy resin. It can also be contained in adhesives.
Description
PPARγ is a ligand-activated transcription factor involved in the regulation of lipid homeostasis and may function as a master regulator of adipogenesis. Ligands for PPARγ include antidiabetic drugs of the thiazolidinedione structural class, 15-deoxy-Δ12,14-prostaglandin J2 , and NSAIDS. BADGE is a synthetic compound used in the production of polycarbonate and industrial plastics. This compound was recently identified as an antagonist of PPARγ. BADGE binds to PPARγ with an apparent Kd of 100 μM and interferes with the ability of 3T3-L1 and 3T3-F442A cells to undergo hormone-mediated cell differentiation.
Chemical properties
Diglycidyl ether of bisphenol A is a colorless to light amber liquid. Slight epoxy odor.
The Uses of BISPHENOL A DIGLYCIDYL ETHER RESIN
Bisphenol A derivative. A PPARγ antagonist; exhibits estrogenic activity; An inhibitor of PPARγ and suppressor of TNFα.
The Uses of BISPHENOL A DIGLYCIDYL ETHER RESIN
In the manufacture of epoxy resins and polycarbonates for food packaging.
The Uses of BISPHENOL A DIGLYCIDYL ETHER RESIN
The uniqueness of D.E.R. 332 epoxy resin is reflected in its maximum epoxide equivalent weight of 178. Because of its high purity and lack of polymer factions, samples embedded using D.E.R. 322 have uniform performance, exceptionally low viscosity and color; and improved elevated temperature properties.
Definition
ChEBI: Bisphenol A diglycidyl ether is a diarylmethane.
Production Methods
The synthesis of the basic epoxy resin molecule involves the reaction of epichlorohydrin with bisphenol A, the latter requiring two basic intermediates for synthesis, acetone and phenol. Theoretically, the production of the bisphenol A diglycidyl ether requires 2 mol of epichlorohydrin for each mole of the phenol. Epoxy resins of higher molecular weight are obtained by reducing the epichlorohydrin/bisphenol A ratio. This reaction involves consumption of the initial epoxy groups in the epichlorohydrin and of some of the groups formed by dehydrohalogenation.
What are the applications of Application
BADGE is an inhibitor of PPARγ and suppressor of TNFα
General Description
Odorless yellowish brown liquid. Sinks in water.
Air & Water Reactions
Oxidizes readily in air to form unstable peroxides that may explode spontaneously [Bretherick 1979 p.151-154, 164]. Insoluble in water.
Reactivity Profile
Epoxides, such as BISPHENOL A DIGLYCIDYL ETHER RESIN, are highly reactive. They polymerize in the presence of catalysts or when heated. These polymerization reactions can be violent. Compounds in this group react with acids, bases, and oxidizing and reducing agents. They react, possibly violently with water in the presence of acid and other catalysts.
Health Hazard
Contact with liquid irritates eyes. Prolonged or repeated contact with skin causes irritation and dermatitis.
Fire Hazard
BISPHENOL A DIGLYCIDYL ETHER RESIN is probably combustible.
Biological Activity
PPAR γ pure antagonist with micromolar affinity in 3T3-L1 and 3T3-F442A preadipocyte cells; selective over PPAR δ and PPAR α . Antagonizes the ability of rosiglitazone to stimulate transcriptional activity of PPAR γ . Acts as a PPAR γ agonist in an ECV304 cell line. Also produces PPARg-independent apoptosis of tumor cells via several mechanisms. Active in vivo .
Contact allergens
Most epoxy resins result from polymerization of bisphenol A diglycidyl ether (BADGE). Delayed hypersensitivity is caused by the low-molecular-weight monomer BADGE (Molecular Weight 340 g/mol), the dimer having much a lower sensitization power. This allergen caused contact dermatitis in six workers in a plant producing printed circuits boards made of copper sheets and fiber glass fabric impregnated with a brominated epoxy resin. It can be contained in adhesives.
Biochem/physiol Actions
PPARγ inhibitor that blocks rosiglitazone- and insulin-induced adipogenesis.
Potential Exposure
Diglycidyl ether of bisphenol A is used as a basic active ingredient of epoxy resins.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.
Carcinogenicity
In summary, a number of carcinogenicity studies involving the topical application of pure BADGE as well as EPON Resin 828 and other commercial BADGE-based resins have been carried out in experimental animals.Viewing the studies as a whole, the weight of evidence does not show that BADGE or BADGE-based epoxy resins are carcinogenic.
Metabolism
Not Available
storage
Color Code—Blue: Health Hazard (skin sensitization): Store in a secure location. Prior to working with thischemical you should be trained on its proper handling andstorage. Store in tightly closed containers in a dark, cool,well-ventilated area away from strong oxidizers, acids, and heat. Recommended storage (and pumping)temperature=45-50℃.
Shipping
This material is not covered in DOT’sPerformance-Oriented Packaging Standards. It may,however, be classified as a Combustible Liquid, n.o.s. Thisclass requires no shipping label. It falls in Hazard Class 3[each reference to a Class 3 material is modified to read“COMBUSTIBLE LIQUID” when that material is reclassified in accordance with y173.150 (e) or (f) of this subchapter or has a flash point above 60.5℃/141°F but below93℃/200°F], and Packing Group III. The symbol “D” identifies proper shipping names which are appropriate fordescribing materials for domestic transportation but may beinappropriate for international transportation under the provisions of international regulations (e.g., IMO, ICAO). Analternate proper shipping name may be selected when eitherdomestic or international transportation is involved.
Incompatibilities
Easily oxidized in air; presumed to form unstable and explosive peroxides in storage. Incompatible with strong acids; strong oxidizers.
Waste Disposal
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber.
References
1) Cuzzocrea?et al. (2004),?Rosiglitazone , a ligand of the peroxisome proliferator-activated receptor-gamma, reduces acute inflammation; Eur. J. Pharmacol.,?483?79 2) Fehlberg?et al. (2003),?Bisphenol A diglycidyl ether-induced apoptosis involves Bax/Bid-dependent mitochondrial release of apoptosis-inducing factor (AIF), cytochrome c and Smac/DIABLO; Br. J. Pharmacol.,?139?495 3) Chamorro-Garcia?et al.?(2012),?Bisphenol A diglycidyl ether induces adipogenic differentiation of multipotent stromal stem cells through a peroxisome proliferator-activated receptor gamma-independent mechanism; Environ. Health Perspect.,?120?984 4) Duque?et al. (2013),?Pharmacological inhibition of PPARgamma increases osteoblastogenesis and bone mass in male C57BL/6 mice; J. Bone Miner. Res.,?28?639
Properties of BISPHENOL A DIGLYCIDYL ETHER RESIN
| Melting point: | 40-44 °C |
| Boiling point: | 210 °C / 1mmHg |
| Density | 1,17 g/cm3 |
| vapor pressure | 0Pa at 25℃ |
| refractive index | 1.5735 |
| storage temp. | Inert atmosphere,2-8°C |
| solubility | Soluble in DMSO (up to 30 mg/ml) or in Ethanol (up to 15 mg/ml) |
| form | viscous liquid |
| color | White |
| Water Solubility | Soluble in 100% ethanol, dimethyl sulfoxide (100 mM), dimethyl formamide, chloroform, methanol, and ethanol (50 mM). Insoluble in water. |
| BRN | 299026 |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20°C for up to 1 month. |
| CAS DataBase Reference | 1675-54-3(CAS DataBase Reference) |
| IARC | 3 (Vol. 47, 71) 1999 |
| EPA Substance Registry System | Bisphenol A diglycidyl ether (1675-54-3) |
Safety information for BISPHENOL A DIGLYCIDYL ETHER RESIN
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H317:Sensitisation, Skin H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P272:Contaminated work clothing should not be allowed out of the workplace. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for BISPHENOL A DIGLYCIDYL ETHER RESIN
New Products
4-Piperidinemethanol Ethyl 2,4-Dihydroxy-6-methylnicotinate Ethyl isonicotinate 3-pyridine methanol N-Methyl 4-chloro-pyridine-2-carboxamide 2-Fluoro-6-iodobenzoic acid 2-((2,6-difluorobenzyl)(ethoxycarbonyl)amino)-4-((dimethylamino)methyl)-5-(4-nitrophenyl)thiophene-3-carboxylic acid Ethyl2-oxo-2,3,9,10-tetrahydro-1H-pyrido[3',4':4,5]pyrrolo[1,2,3-de]quinoxaline-8(7H)-carboxylate Elinzanetant tert-butyl 2-(4-amino-6-chloropyrimidin-5-yloxy)ethylmethylcarbamate Phenylazomalononitrile 5,6 Dimethoxy-1-indanone 3-Iodophenylacetic acid 2-Hexyn-1-ol Dibenzo-18-crown-6 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-Pyridineacetonitrile, α-hydroxy- 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole N Ethylmethylamine Ethyl Methanesulfonate N N' DimethylEthylenediamine Lead II Bromide Variamine Blue B Diazonium salt N N N'Trimethyl ethylenediamineRelated products of tetrahydrofuran





You may like
-
 Bisphenol A diglycidyl ether CAS 1675-54-3View Details
Bisphenol A diglycidyl ether CAS 1675-54-3View Details
1675-54-3 -
 Bisphenol A diglycidyl ether CAS 1675-54-3View Details
Bisphenol A diglycidyl ether CAS 1675-54-3View Details
1675-54-3 -
 Bisphenol a diglycidyl ether 97% CAS 1675-54-3View Details
Bisphenol a diglycidyl ether 97% CAS 1675-54-3View Details
1675-54-3 -
 2,2-Bis(4-glycidyloxyphenyl)propane CAS 1675-54-3View Details
2,2-Bis(4-glycidyloxyphenyl)propane CAS 1675-54-3View Details
1675-54-3 -
 Bisphenol A diglycidyl ether CAS 1675-54-3View Details
Bisphenol A diglycidyl ether CAS 1675-54-3View Details
1675-54-3 -
 Bisphenol A diglycidyl ether CAS 1675-54-3View Details
Bisphenol A diglycidyl ether CAS 1675-54-3View Details
1675-54-3 -
 D.E.R.™ 332 CAS 1675-54-3View Details
D.E.R.™ 332 CAS 1675-54-3View Details
1675-54-3 -
 5162-90-3 2-Amino-3-(1,2-dihydro-2-oxoquinoline-4-yl)propanoic acid 97%View Details
5162-90-3 2-Amino-3-(1,2-dihydro-2-oxoquinoline-4-yl)propanoic acid 97%View Details
5162-90-3
