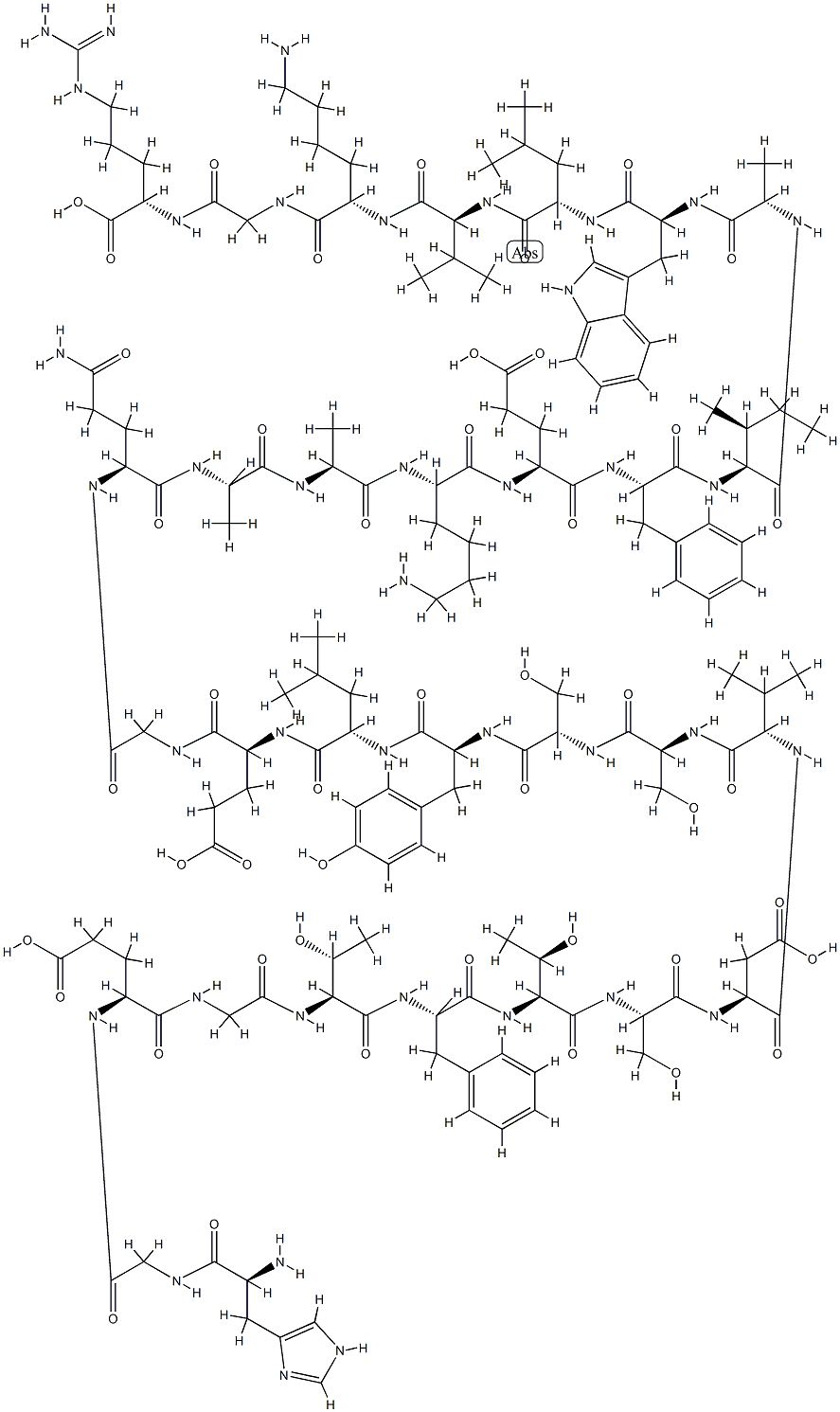
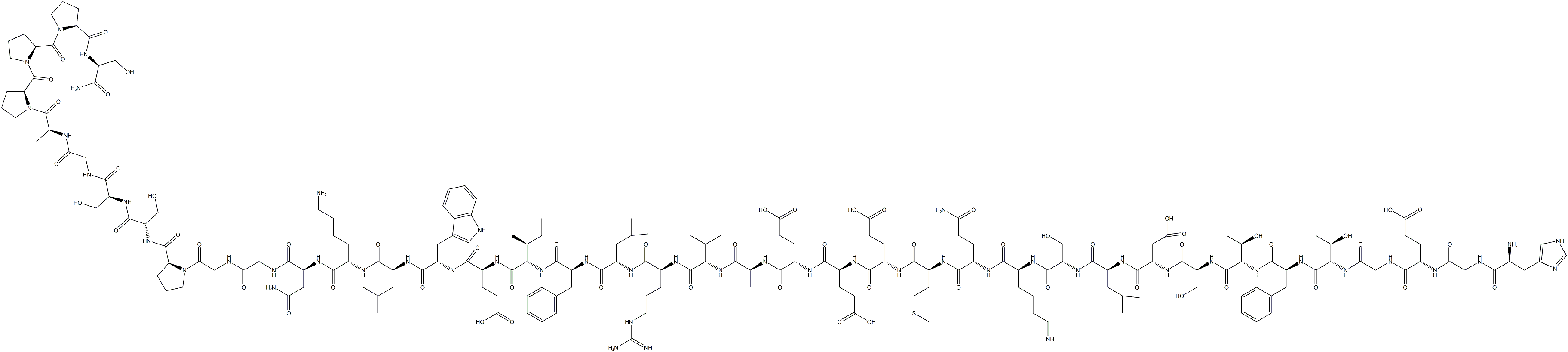
GLP-1
Synonym(s):GLP-1;Preproglucagon 72-108
- CAS NO.:87805-34-3
- Empirical Formula: C186H275N51O59
- Molecular Weight: 4169.48
- MDL number: MFCD00131174
- EINECS: 201-258-5
- Update Date: 2025-11-22 16:52:42
What is GLP-1?
Description
GLP-1 stimulates pancreatic β cells to secrete insulin as an incretin hormone. GLP-1 also has additional actions, including suppression of glucagon secretion, inhibition of gastric motility, and promotion of satiety.
History
GLP-1 was first identified in the early 1980s, following the cloning of the proglucagon gene. The biological activity of GLP-1 was investigated initially by using the N-terminal extended forms of GLP-1(1–37 and 1–36 amide). However, these forms were devoid of biological activity. In the late 1980s, bioactive GLP-1 peptides were purified from gut extracts and were identified to be GLP1(7–37) and GLP-1(7–36)-amide. In the current literature, the unqualified designation of GLP-1 corresponds to the truncated peptides.
The Uses of GLP-1
Glucagon-Like Peptide I (GLP1) has been used as an antigen in excess amounts for the incubation of primary antibodies, post-immunocytochemistry with positive results of paraffin-embedded pancreatic tissues of Rana temporaria.
Biosynthesis
Glucagon, GLP-1, and GLP-2 are processed from proglucagon in pancreatic α cells and intestinal L cells in a tissue-specific manner. Prohormone convertases (PCs) are responsible for the tissue-specific processing. Glucagon is liberated from pancreatic α cells by PC2, whereas GLP-1 and GLP-2 are liberated from the intestinal L cells by PC1/3. As a result, GLP-1 and GLP-2 are coreleased in a 1:1 ratio following nutrient ingestion. The secretion of GLP-1 is stimulated by nutrients such as lipids and carbohydrates. In addition, several hormones such as cholecystokinin (CCK), GIP, and somatostatin as well as numerous neuromediators regulate GLP-1 secretion.2 Insulin has been reported to inhibit GLP-1 release, indicating a feedback regulation of GLP-1 secretion.
General Description
Glucagon-Like Peptide I (GLP1) is an incretin hormone, which functions as ligand for GLP1 receptors (GLP1R). These receptors are expressed in renal and cardiovascular system, such as smooth muscle and endothelial cells.
Biochem/physiol Actions
Glucagon-Like Peptide I (GLP1) controls the metabolism of glucose, by inducing the secretion of insulin and suppressing that of glucagon. Studies in Indonesian population show that reduced levels of this protein are linked with increased susceptibility to type 2 diabetes mellitus. Plasma levels of this hormone also show significant association with systolic and diastolic blood pressure levels.
Clinical Use
GLP-1 enhances glucose-stimulated insulin release. GLP-1 agonists are resistant to degradation by DPP-4; thus these agonists are used clinically for the treatment of diabetes. DPP-4 inhibitors are also acceptable for use in diabetes patients because GLP-1 is rapidly deactivated by DPP-4.
Side Effects
Common side effects of GLP-1 include: loss of appetite, nausea, vomiting, diarrhoea. Other side effects may include: dizziness, mild tachycardia or increased heart rate, infection, headache, and indigestion. Temporary mild itching and redness of the skin at the injection site may also occur. Serious and rare side effects include: pancreatitis, medullary thyroid cancer, acute (sudden) kidney injury, worsening of diabetes-related retinopathy, and severe allergic reactions. And GLP-1 agonists are recommended during pregnancy because they can cause abnormal foetal development. Seek medical attention if any serious adverse reactions occur.
Receptors
Structure and subtype
The receptor of GLP-1 (GLP1R) is a seventransmembrane GPCR that belongs to a subclass of the family B. The GLP-1 receptor was first identified by ligand binding experiments and measurements of cyclic AMP accumulation using the rat insulinoma cell line.3 The human GLP-1 receptor gene, GLP1R, is located on chromosome 6 (6p21). The GLP-1 receptor contains a large hydrophilic extracellular domain and seven hydrophobic transmembrane domains. In addition, the GLP-1 receptor has three potential N-linked glycosylation sites, and glycosylation may modulate the receptor function.
Signal transduction pathway
The GLP-1 receptor is functionally coupled to Gs. 3 Through the activation of AC, cAMP is formed and activates PKA.Ligand stimulation of the receptor alsoincreases the cytoplasmic concentration of Ca2+; this is brought through the Na+ -dependent uptake of extracellular Ca2+ and through the release of Ca2+ from intracellular Ca2+ stores. The increased cytosolic Ca2+ in conjunction with the activated PKA stimulates the translocation and exocytosis of insulin-containing secretory granules.
Agonists
Exendin-4 (subsequently renamed exenatide, the first FDA approved “incretin mimetic”), Liraglutide (a longacting DPP-4-resistant GLP-1 receptor agonist), CJC1131 (a GLP-1 analog engineered for covalent coupling to albumin), Albiglutide (originally referred to as albugon, a recombinant human albumin-GLP-1 protein), ZP10 (an exendin-4 derivative), BIM51077 (subsequently renamed taspoglutide), LY315902 (a DPP-4-resistant GLP-1 analog), LY2428757 (a pegylated GLP-1), LY2199265 (Dulaglutide, an Fc immunoglobulin fusion protein), Semaglutide (a synthetic peptide similar to GLP-1), Lixisenatide (a synthetic peptide similar to exandin-4), and Tirzepatide (LY3298176, a dual agonist of GIP and GLP-1 receptors).
Antagonists
Exendin (9–39), T-0632.
Storage
Store at -20°C
Structure and conformation
GLP-1 is a 31-aa peptide hormone and is secreted from intestinal endocrine L cells in two major molecular forms, GLP-1(7–37) and GLP-1(7–36)-amide. Amidation is not always important for its biological activity, and these two molecular forms have similar biological activities. The majority of circulating GLP-1 is found to be GLP-1(7–36) amide, with lesser amounts of GLP-1 (7–37). GLP-1 shares a highly conserved alanine at position 9 with GIP and GLP-2, making these peptides ideal substrates for dipeptidyl peptidase 4 (DPP-4).
The primary structure of GLP-1 is highly conserved in most mammals, except for the platypus (11 substitutions). In the platypus and squirrel, GLP-1 has one substitution of the C-terminal glutamate.
Mode of action
Glucagon-like peptide-1 (GLP-1) released from gut enteroendocrine cells controls meal-related glycemic excursions through augmentation of insulin and inhibition of glucagon secretion. GLP-1 also inhibits gastric emptying and food intake, actions maximizing nutrient absorption while limiting weight gain.
Properties of GLP-1
| storage temp. | −20°C |
| form | Solid |
| Water Solubility | Soluble to 5 mg/ml in water |
| CAS DataBase Reference | 87805-34-3 |
Safety information for GLP-1
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for GLP-1
| InChIKey | UKVFVQPAANCXIL-FJVFSOETSA-N |
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
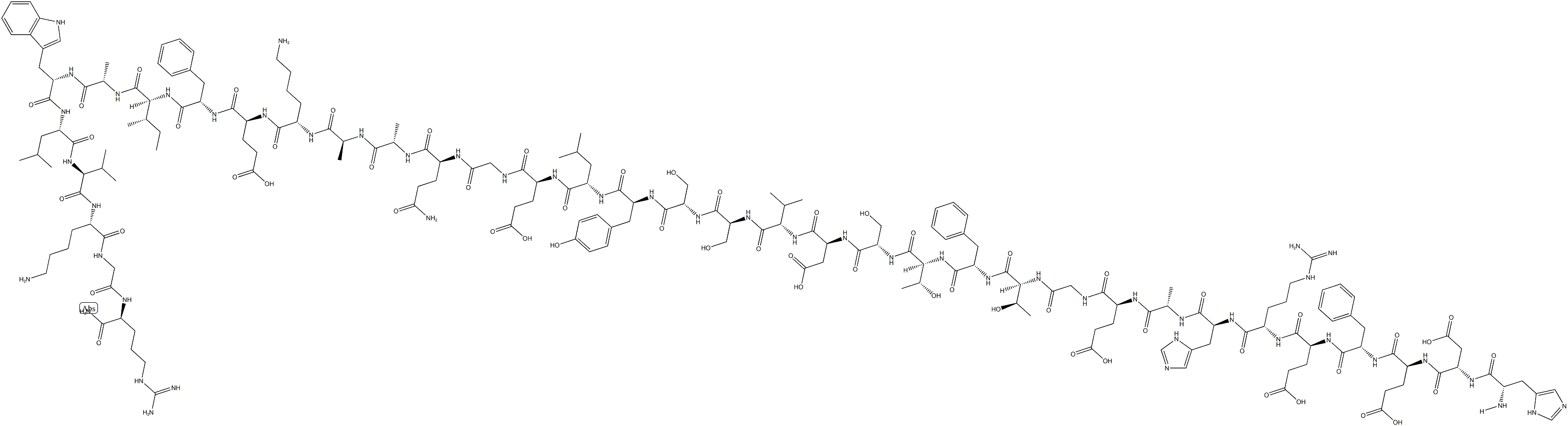
![GLP-1 [7-36]](https://img.chemicalbook.in/CAS/GIF/107444-51-9.gif)

You may like
-
 Glucagon-Like Peptide I human CAS 87805-34-3View Details
Glucagon-Like Peptide I human CAS 87805-34-3View Details
87805-34-3 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
