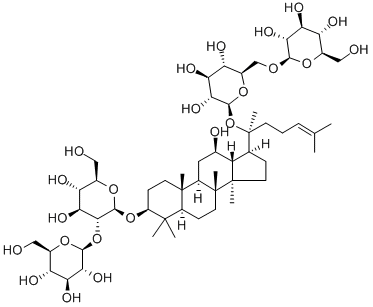
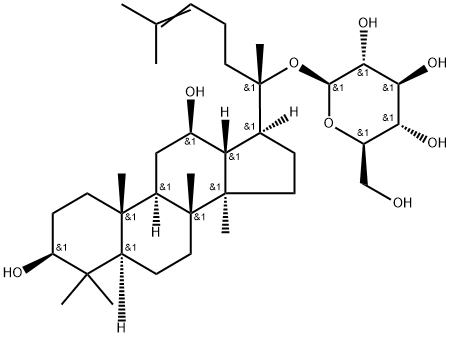
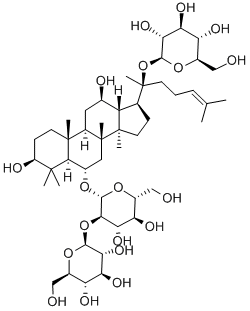
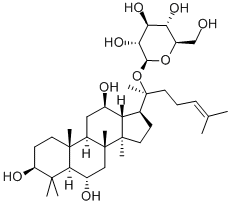
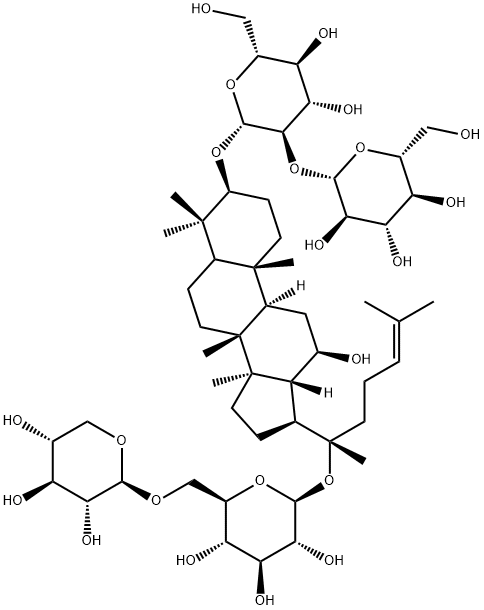
Ginsenoside Rg1
Synonym(s):Ginsenoside Rg1;Sanchinoside C1;Ginsenoside A2;Ginsenoside g1;Panaxoside A
- CAS NO.:22427-39-0
- Empirical Formula: C42H72O14
- Molecular Weight: 801.01
- MDL number: MFCD00210293
- EINECS: 244-989-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
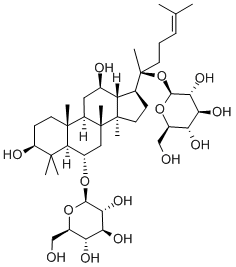
What is Ginsenoside Rg1?
Description
Ginsenoside RG1(Rg1) is a natural product found in Panax notoginseng, Panax ginseng, and Panax vietnamensis. It is one of the primary bioactive ingredients in Panax notoginseng saponins. Numerous studies have indicated that Rg1 plays significant roles in health benefits such as anticancer, neuroprotective, anti-senescence, anti-inflammation, and antioxidative effects. Also, extensive research has demonstrated that Rg1 is associated with various diseases, including cardiovascular disease, Alzheimer’s disease, spinal cord injury, and liver injury. Xue et al. and Yu et al. demonstrated that Rg1 ameliorated streptozotocin-induced oxidative stress and myocardial apoptosis and suppressed HG-induced mesenchymal activation and fibrosis[1].
Chemical properties
White solid
The Uses of Ginsenoside Rg1
Ginsenoside Rg1 protects against arthitis through osteoclast differentiation inhibition. Anti-tumor agent, inhibits the NF-kB-dependant MMP-expression in PMA (phorbol myristate acetate)-induced tumor cell invasion.
What are the applications of Application
Ginsenoside Rg1 is a class of triterpene saponins and steroid glycosides
Definition
ChEBI: A ginsenoside found in Panax ginseng and Panax japonicus var. major that is dammarane which is substituted by hydroxy groups at the 3beta, 6alpha, 12beta and 20 <ital pro-S positions, in which the hydroxy groups at positions 6 and 20 have been converted to the corresponding beta-D-glucopyranosides, and in which a double bond has been introduced at the 24-25 posit on.
References
[1] Hui Liu. “Ginsenoside Rg1 attenuates the inflammation and oxidative stress induced by diabetic nephropathy through regulating the PI3K/AKT/FOXO3 pathway.” Annals of translational medicine 9 24 (2021): 1789.
Properties of Ginsenoside Rg1
| Melting point: | 194~197℃ |
| Boiling point: | 898.5±65.0 °C(Predicted) |
| Density | 1.33±0.1 g/cm3(Predicted) |
| storage temp. | Sealed in dry,Store in freezer, under -20°C |
| solubility | DMSO (Slightly), Methanol (Slightly), Pyridine (Slightly) |
| form | neat |
| pka | 12.91±0.70(Predicted) |
| form | Solid |
| color | White to Off-White |
| Stability: | Hygroscopic |
| CAS DataBase Reference | 22427-39-0(CAS DataBase Reference) |
Safety information for Ginsenoside Rg1
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P501:Dispose of contents/container to..… |
Computed Descriptors for Ginsenoside Rg1
| InChIKey | YURJSTAIMNSZAE-OLCOBRLXNA-N |
New Products
3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-Iodophenylacetic acid Cyclohexane, (2-propynyloxy)- (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine Pivalic anhydride,98% Phenylmethanesulfonyl fluoride, 98% Glyoxylic acid solution, 50% in water tert-Butyl glycinate,97% 4-Ethoxybenzoic acid, 99% Sodium 1-octanesulfonate monohydrate 7-Ethyl Tryptophol 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl N, N-Carbonyldiimidazole (CDI) 5-Cyanophthalide 10-Methoxy-5H-dibenz[b,f]azepine 3-Methoxybenzonitrile Dibenzoyl Peroxide 4-Methoxybenzonitrile Titanium Dioxide Chloral PentachlorobenzonitrileRelated products of tetrahydrofuran
You may like
-
 Ginsenoside Rg1 96% CAS 22427-39-0View Details
Ginsenoside Rg1 96% CAS 22427-39-0View Details
22427-39-0 -
 Ginsenoside Rg1 CAS 22427-39-0View Details
Ginsenoside Rg1 CAS 22427-39-0View Details
22427-39-0 -
 Avibactam INT 1View Details
Avibactam INT 1View Details
1416134-48-9 -
 Avibactam sodium seriesView Details
Avibactam sodium seriesView Details
1192651-49-2 -
 Avibactam seriesView Details
Avibactam seriesView Details
1192651-80-1 -
 Avibactam Sodium SaltView Details
Avibactam Sodium SaltView Details
1192491-61-4 -
 Avibactam intermediateView Details
Avibactam intermediateView Details
1171080-45-7 -
 Relibatan intermediateView Details
Relibatan intermediateView Details
1416134-63-8
