Garlic oil
- CAS NO.:8008-99-9
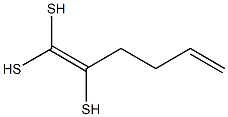
- Empirical Formula: C6H10S3
- Molecular Weight: 178.3386
- MDL number: MFCD00240715
- EINECS: 232-371-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:50:40
What is Garlic oil?
Chemical properties
Garlic oil is obtained by steam distillation of crushed bulbs of the common garlic, Allium sativum L.; it is produced mainly in China, Mexico, India, Egypt, and, on a smaller scale, also in Europe (France,Hungary) and is a clear, reddish-orange liquid, with a strong, pungent, characteristic garlic odor.
Chemical properties
Garlic is an herbaceous, perennial plant of Mediterranean origin Its stalk can reach lengths of 1 m (39 in.) The plant exhibits fat, keeled leaves and is terminated by an umbellated fower cluster The bulb-like root contains several bulbils (cloves) enclosed in a common membrane The bulbs are the part used Garlic has a pungent, acrid, aromatic, garlic-like odor In addition to the natural form, garlic is marketed as minced dehydrated garlic; garlic powder, which is ground dehydrated cloves; garlic salt, which is garlic powder mixed with table salt, and, if necessary, some edible starch to prevent caking; and oil of garlic, which is steam- distilled from crushed garlic bulbs.
Occurrence
Garlic is a perennial bulb found throughout the world
The Uses of Garlic oil
garlic oil is considered a healing oil with bacteriostatic and bactericidal action. It is incorporated into skin care formulations for skin healing and to aid in clearing problems such as eczema and acne.
Definition
Extractives and their physically modified derivatives such as tinctures, concretes, absolutes, essential oils, oleoresins, terpenes, terpene-free fractions, distillates, residues, etc., obtained from Allium sativum, Liliaceae.
Essential oil composition
Although found in only small quantities, fresh garlic is a known source of several vitamins, minerals and trace elements Garlic is known to contain the highest sulfur content of any member of the allium genus The bulbs contain a colorless and odorless sulfur-containing amino acid called alliin (S-allyl-L-cysteine sulfoxide) Major chemical constituents of whole garlic include alliin (allylsulfnyl alanine), which is rapidly converted enzymatically when garlic is crushed to allicin (responsible for the characteristic odor of the essential oil and for the odor liberated from the crushed garlic clove), volatile and fatty acids, mucilage and albumin.
Taste threshold values
Taste characteristics at 0.15 ppm: sweet, garlic, cooked brown and sauteed garlic.
General Description
Garlic (Allium sativum) is an herbal drug with references to medicinal properties that date back thousands of years. Of all the herbal remedies available to consumers, garlic is probably the most extensively researched.
Garlic contains a key component, alliin or S-methyl-lcysteine sulfoxide. Additionally, methiin (methylcysteine sulfoxide), cycloalliin, several γ-L-glutamyl-S-alkyl-Lcysteines, and alkyl alkanethiosulfinates are present. The tissues also contain enzymes (alliinase, peroxidase, myrosinase), as well as additional sulfur-containing compounds, ajoene, and other minor components.
Garlic itself has the highest sulfur content of all of the Allium species. When the garlic clove is crushed, the enzyme alliinase is released, and alliin is converted to allicin. Allicin gives garlic its characteristic odor and is believed to be the pharmacologically active ingredient from the herb. Alliinase will not survive the acidic environment of the stomach, so enteric-coated tablets are used. Garlic is most often studied for its lipid-lowering and antithrombotic effects. A cholesterol-lowering effect is well documented in both animals and humans. Garlic lowers serum cholesterol, triglycerides, and low-density lipoprotein (LDL) while increasing high-density lipoprotein (HDL).
Anticancer Research
For many centuries, garlic has been used to treat a range of illnesses (Gupta et al.2004). Garlic oil contains a sulphur-holding substance known as ajoene oil or allicin.This oil has been found to control cancer cell production. The anticancer activityof garlic is thus due to its high levels of organic sulphides and polysulphides. Themechanism behind the antitumour activity triggered by stimulating the lymphocytesand macrophages is that they kill cancerous cells and interfere with tumour cells’metabolism. The administration method involves taking 5–6 cloves of crushed garlic,around 5 g/day (Kapoor 2000). The crushed cloves should sit for at least 15 minto release the allinase enzyme before consumption (Shareef et al. 2016).
Properties of Garlic oil
| Boiling point: | 150-208 °C |
| Density | 1.083 g/mL at 25 °C |
| refractive index | n20/D 1.575 |
| Flash point: | 47 °C |
| solubility | Chloroform, Methanol (Slightly) |
| form | Oil |
| color | Clear Colourless |
| Odor | at 0.10 % in propylene glycol. garlic |
| InChI | InChI=1S/C6H10S3/c1-2-3-4-5(7)6(8)9/h2,7-9H,1,3-4H2 |
| EPA Substance Registry System | Garlic, ext. (8008-99-9) |
Safety information for Garlic oil
Computed Descriptors for Garlic oil
| InChIKey | XGYVEKBQAPYNLL-UHFFFAOYSA-N |
| SMILES | C(/S)(\S)=C(/S)\CCC=C |
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran







You may like
-
 Garlic Oil 99%View Details
Garlic Oil 99%View Details -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
