Furaneol
Synonym(s):2,5-Dimethyl-4-hydroxy-3(2H)-furanone;Furaneol;Strawberry furanone
- CAS NO.:3658-77-3
- Empirical Formula: C6H8O3
- Molecular Weight: 128.13
- MDL number: MFCD00010706
- EINECS: 222-908-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:22
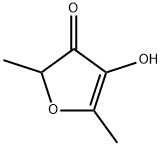
What is Furaneol?
Description
Furaneol is a very important aroma chemical and is a key flavoring compound found in many kinds of fruits. It can be chemically formed through different carbohydrates during the Mainlard reaction. It can also be synthesized by yeast, bacteria and plants and play some physiological effects. Study has shown that it can suppress the phosphorylation of cAMP response element binding protein, underlying its potential role as an effective inhibitor of hyperpigmentation. It also has the potential to become an effective antimicrobial agent for human beings.
Description
Furaneol is a natural compound with oxygen atoms in ketone, hydroxyl, and ether functional groups. It is associated primarily with the odor of strawberries, but it is also found in food crops as diverse as pineapple, tomato, and buckwheat.
Furaneol contains an asymmetric carbon atom and therefore exists as two enantiomers. The (R)-enantiomer (shown) is the one found in nature; it imparts a much stronger odor (and flavor) to foods than its (S) counterpart.
Methoxyfuraneol (furaneol with a methyl group in place of the hydroxyl hydrogen atom) is also an important strawberry aroma constituent. Both molecules are used in strawberry-scented perfumes and flavoring agents.
Chemical properties
white to light yellow crystal powde
Chemical properties
Furaneol is a constituent of pineapple and strawberry aroma and is also found in other foods. It forms colorless crystals (mp 77–79°C) with a relatively weak, nonspecific odor. Dilute solutions develop a pineapple, strawberrylike odor.
Chemical properties
4-Hydroxy-2,5-dimethyl-3(2H)-furanone has a fruity caramel or "burnt pineapple" aroma. May be synthesized from dimethyl- 3,4-dihyroxyfuran-2,5-dicarboxylate.
Chemical properties
Furaneol has a sweet, fruity, strawberry, hot sugar, fruity caramel or “burnt pineapple” aroma.
Occurrence
Reported found in guava, grapes, pineapple, raspberry, strawberry fruit and jam, rye bread, Swiss cheese, boiled beef, beer, cocoa, coffee, tea, filberts, almonds, oatmeal, Arctic bramble, yellow passion fruit, mango, shoyu, fermented soy sauce, litchi, malt and Cape gooseberry.
The Uses of Furaneol
2,5-Dimethyl-4-hydroxy-3(2H)-furanone is a component of meat essence composition. 2,5-Dimethyl-4-hydroxy-3(2H)-furanone is used in the flavor and perfume industry due to its sweet strawberry aroma.
Definition
ChEBI: A member of the class of furans that is 2,5-dimethylfuran carrying additional oxo and hydroxy groups at positions 3 and 4 respectively. It has been found particularly in strawberries and other such fruits.
Preparation
Furaneol can be prepared by cyclization of hexane-2,5-diol-3,4-dione in the presence of an acidic catalyst.The dione is the ozonization product of 2,5- hexynediol, which is obtained by ethynylation of acetaldehyde.
In another process, a dialkyl ??-methyldiglycolate (formed from an alkyl lactate and an alkyl monochloroacetate) is reacted with dialkyl oxalate in the presence of a sodium alkoxide and dimethylformamide. The reaction product is cyclized, alkylated, hydrolyzed, and decarboxylated.In another process, a dialkyl ??-methyldiglycolate (formed from an alkyl lactate and an alkyl monochloroacetate) is reacted with dialkyl oxalate in the presence of a sodium alkoxide and dimethylformamide. The reaction product is cyclized, alkylated, hydrolyzed, and decarboxylated .
Aroma threshold values
Detection: 0.03 to 60 ppb; aroma characteristics at 0.1%: sweet, slightly burnt brown caramellic, cotton candy with a savory nuance
Taste threshold values
Taste characteristics at 0.10 to 1.0 ppm: sweet caramellic cooked meaty and fruity nuances
Synthesis Reference(s)
The Journal of Organic Chemistry, 57, p. 5023, 1992 DOI: 10.1021/jo00044a047
Synthesis, p. 377, 1987
General Description
4-Hydroxy-2,5-dimethyl-3(2H)-furanone (HDMF, DMHF) is a caramel-like smelling compound, identified in Maillard reaction systems based on pentoses by GC-MS and GCMS/MS. Solubility data of DMHF in six different solvents over the temperature range from 283.15K to 313.15K under atmospheric pressure of 0.10 MPa has been examined by dynamic method. Separation of the enantiomers of DMHF by capillary electrophoretic method has been reported. Commercially it is synthesized from L-rhamnose. DMHF has been identified as acidic odorant in Thai premium fish sauce samples by aroma extract dilution analysis (AEDA). It is identified as key aroma compound in the distiller′s grains (DG) from wheat. It is reported as the main flavor compound in strawberries and its biosynthesis has been reported. HDMF has been found in various fruits such as pineapples strawberries and grapes, as well as in beef broth , roasted coffee, bread crust, roasted beef, roasted sesame seeds and stewed beef.
Flammability and Explosibility
Not classified
Trade name
Furaneol® (Firmenich).
Biochem/physiol Actions
Taste at 0.10 to 1.0 ppm
Synthesis
From dimethyl-3,4-dihydroxyfuran-2,5-dicarboxylate
References
Pickenhagen, Wilhelm, et al. "Estimation of 2,5-dimethyl-4-hydroxy-3(2H)-furanone (FURANEOLA®) in cultivated and wild strawberries, pineapples and mangoes." Journal of the Science of Food & Agriculture 32.11(2010):1132-1134.
Farine, Jean Pierre, et al. "4-Hydroxy-5-methyl-3(2H)-furanone and 4-Hydroxy-2,5-dimethyl-3(2H)-furanone, Two Components of the Male Sex Pheromone of Eurycotis floridana (Walker) (Insecta, Blattidae, Polyzosteriinae)." Bioscience Biotechnology & Biochemistry 57.12(2014):2026-2030.
Schwab, W. "Natural 4-hydroxy-2,5-dimethyl-3(2H)-furanone (Furaneol®). " Molecules 18.6(2013):6936-51.
Properties of Furaneol
| Melting point: | 73-77 °C(lit.) |
| Boiling point: | 188 °C |
| Density | 1.049 g/mL at 25 °C |
| vapor pressure | 0.81Pa at 25℃ |
| refractive index | n |
| FEMA | 3174 | 4-HYDROXY-2,5-DIMETHYL-3(2H)-FURANONE |
| Flash point: | >230 °F |
| storage temp. | 2-8°C |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | neat |
| pka | 9.62±0.40(Predicted) |
| form | Solid |
| color | White |
| Odor | at 1.00 % in propylene glycol. sweet cotton candy caramel strawberry sugar |
| Water Solubility | 176g/L at 20℃ |
| JECFA Number | 1446 |
| BRN | 1281357 |
| Stability: | Light Sensitive, Temperature Sensitive |
| CAS DataBase Reference | 3658-77-3(CAS DataBase Reference) |
| NIST Chemistry Reference | 2,5-Dimethyl-4-hydroxy-3(2h)-furanone(3658-77-3) |
| EPA Substance Registry System | 4-Hydroxy-2,5-dimethyl-3(2H)furanone (3658-77-3) |
Safety information for Furaneol
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H314:Skin corrosion/irritation H317:Sensitisation, Skin |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Furaneol
| InChIKey | INAXVXBDKKUCGI-UHFFFAOYSA-N |
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 3658-77-3 Furaneol 98%View Details
3658-77-3 Furaneol 98%View Details
3658-77-3 -
 2,5-Dimethyl-4-hydroxy-3(2H)-furanone CAS 3658-77-3View Details
2,5-Dimethyl-4-hydroxy-3(2H)-furanone CAS 3658-77-3View Details
3658-77-3 -
 2,5-Dimethyl-4-hydroxy-3(2H)-furanone (15% in Propylene Glycol, ca. 1.2mol/L) CAS 3658-77-3View Details
2,5-Dimethyl-4-hydroxy-3(2H)-furanone (15% in Propylene Glycol, ca. 1.2mol/L) CAS 3658-77-3View Details
3658-77-3 -
 4-Hydroxy-2,5-dimethyl-3(2H)-furanone CAS 3658-77-3View Details
4-Hydroxy-2,5-dimethyl-3(2H)-furanone CAS 3658-77-3View Details
3658-77-3 -
 4-Hydroxy-2,5-dimethyl-3(2H)-furanone CAS 3658-77-3View Details
4-Hydroxy-2,5-dimethyl-3(2H)-furanone CAS 3658-77-3View Details
3658-77-3 -
 Furaneol (3658-77-3) (C6H8O3), Grade: Industrial Grade, Purity: 98%View Details
Furaneol (3658-77-3) (C6H8O3), Grade: Industrial Grade, Purity: 98%View Details
3658-77-3 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
