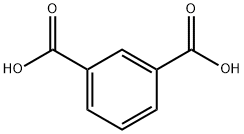
2,5-Furandicarboxylic acid
Synonym(s):Dehydromucic acid;FDCA
- CAS NO.:3238-40-2
- Empirical Formula: C6H4O5
- Molecular Weight: 156.09
- MDL number: MFCD00016582
- EINECS: 221-800-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
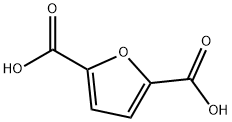
What is 2,5-Furandicarboxylic acid?
Description
2,5-Furandicarboxylic acid (FDCA) is an organic chemical compound consisting of two carboxylic acid groups attached to a central furan ring. It was first reported as dehydromucic acid by Rudolph Fittig and Heinzelmann in 1876, who produced it via the action of concentrated hydrobromic acid upon mucic acid. It can be produced from certain carbohydrates and as such is a renewable resource, it was identified by the US Department of Energy as one of 12 priority chemicals for establishing the “green” chemistry industry of the future.Furan-2,5-dicarboxylic acid (FDCA) has been suggested as an important renewable building block because it can substitute for terephthalic acid (PTA) in the production of polyesters and other current polymers containing an aromatic moiety.
wikipedia
Description
2,5-Furandicarboxylic acid (FDCA) is recognised as a potentially sustainable monomer for polyester production. It can be made from acyclic galactose (mucilage) acid of sugar or from 5-(hydroxymethyl)furfural and 5-(methoxymethyl)furfural. FDCA can be used in conjunction with biobased glycols for the manufacture of sustainable poly(ethylene furanate) (PEF), a polyester with properties similar to those of the widely-used polyethylene terephthalate (PET). FDCA is also used in the production of polyesters, such as poly(vinylfuran), which is used in the manufacture of polyethylene terephthalate. In fact, PEF offers a better gas barrier than PET, making it an excellent candidate for the manufacture of plastic bottles for carbonated beverages.
Chemical properties
2,5-Furandicarboxylic acid is a chemical intermediate with high sensitivity and good stability. It is soluble in water under alkaline conditions and is a white powder solid under acidic conditions, and is an important monomer for the preparation of corrosion-resistant plastics. It is irritating to eyes, respiratory tract and skin.
History
2,5-Furandicarboxylic acid, also known as dehydromucic acid, is a furan derivative. This organic compound was first obtained by Fittig and Heinzelmann in 1876. More than 125 years later, FDCA was identified by the US Department of Energy as one of 12 priority chemicals for establishing the "green" chemistry industry of the future. On a laboratory scale, it is often synthesized from 5- hydroxymethylfurfural (HMF), which in turn can be obtained from carbohydrate-containing sources such as glucose, fructose, sucrose, and starch. From fructose and glucose, HMF is obtained by acidic eliminating three moles of water.
The Uses of 2,5-Furandicarboxylic acid
Interest in renewable based polymers has led to 2,5-furandicarboxylic acid being proposed as a green, sustainable alternative to the widely used petroleum-based terephthalic acid in the synthesis of polyesters. 2,5-Furandicarboxylic acid is produced from oxidation of 5-hydroxymethylfurfural (HMF) which is obtained from the dehydration of bio-based sugars such as fructose.
Definition
ChEBI: A member of the class of furans carrying two carboxy substituents at positions 2 and 5.
What are the applications of Application
2,5-Furandicarboxylic acid (FDCA) is a renewable, greener substitute for terephthalate in the production of polyesters. It is widely used as a precursor for the synthesis of bio-based polyesters and various other polymers.
Applications of FDCA in the synthesis of several metal-organic frameworks (MOFs) have also been reported.
Preparation
2,5-Furandicarboxylic acid (FDCA) can be produced from biomass or its derived sugars or platform chemicals, and has demonstrated to be a very promising substitute for petroleum-derived polymer products.
Chapter 5 - Advances in the synthesis and application of 2,5-furandicarboxylic acid
Synthesis Reference(s)
Tetrahedron Letters, 26, p. 1777, 1985 DOI: 10.1016/S0040-4039(00)98336-9
Flammability and Explosibility
Not classified
Solubility in organics
2,5-Furandicarboxylic acid (FDCA) serves as a monomer in various polyesters and is often obtained through the oxidation of 5-hydroxymethylfurfural. In pure solvents and binary mixtures, the solubility of FDCA increased with the increasing temperature. The order from largest to smallest solubility in pure solvents was as follows: methanol, 1-butanol, isobutanol, acetic acid, water, MIBK, ethyl acetate, and acetonitrile.
https://doi.org/10.1021/acs.jced.7b00927
Properties of 2,5-Furandicarboxylic acid
| Melting point: | >300 °C |
| Boiling point: | 240.29°C (rough estimate) |
| Density | 1.7400 |
| refractive index | 1.6400 (estimate) |
| storage temp. | Keep in dark place,Sealed in dry,Room Temperature |
| solubility | DMSO (Slightly), Methanol (Slightly Sonicated) |
| pka | 2.28±0.10(Predicted) |
| form | Solid |
| appearance | white solid |
| color | White to Dark Brown |
| Water Solubility | 1g/L(18 ºC) |
| Stability: | Light Sensitive, Very Hygroscopic |
| CAS DataBase Reference | 3238-40-2(CAS DataBase Reference) |
| NIST Chemistry Reference | Furan-2,5-dicarboxylic acid(3238-40-2) |
| EPA Substance Registry System | 2,5-Furandicarboxylic acid (3238-40-2) |
Safety information for 2,5-Furandicarboxylic acid
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for 2,5-Furandicarboxylic acid
| InChIKey | CHTHALBTIRVDBM-UHFFFAOYSA-N |
Abamectin manufacturer
JSK Chemicals
Meenaakshi Molecules Pvt Ltd
AVIGYABIOSCIENCES PRIVATE LIMITED
ARRKEM LIFE SCIENCES PRIVATE LIMITED
Dr R R Organics
Maithili Life Sciences Pvt Ltd
BASR Fine Chemicals Pvt. Ltd.
New Products
4-Fluorophenylacetic acid (S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid 5-Aminoimidazole-4-Carbonitrile 4-chloro-3,5-dinitropyridine 2'-Methoxy-biphenyl-2-carboxaldehyde 2-(2-Aminoethyl)isothiourea dihydrobromide, 1-(4-chlorophenyl)propan-1-one 2-Ethyl-4-methyl-1-pentanol DIISOPROPYL MALONATE PENTAFLUOROPHENOL 2-Aminonicotinic acid 6-(4-AMINOPHENYL)-5-METHYL-4,5-DIHYDRO-3(2 H)-PYRDAZINONE β-BUTYROLACTONE 3-OXO-CYCLOBUTANECARBOXYLIC ACID 3-methyl xanthine 1H-Pyrazole-3-carboxylic acid [1,1'-Biphenyl]-4-carboxylic acid (3aR,4R,5R,6aS)-hexahydro-2-oxo-4-[(1E)-3-oxo-4-[3- (trifluoromethyl)phenoxy]-1-buten-1-yl]-2H-cyclopenta[b]furan-5-yl ester 2H-Cyclopenta[b]furan-2,5-diol, hexahydro-4-[(1E,3R)-3-hydroxy-4-[3-(trifluoromethyl)phenoxy]-1- buten-1-yl]-, (3aR,4R,5R,6aS)- 2,5-Dibromopyridine Dimethyl (2-oxo-4-phenylbutyl)phosphonate S-(2-Chloro-3-nitrophenyl) O-ethyl carbonodithioate 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carboxylic acid 2,4-Dichloro-1-[2-nitro-4-(trifluoromethyl)phenoxy]benzeneRelated products of tetrahydrofuran
You may like
-
 Furan 2,5-dicarboxylic-acid 3238-40-2 98%View Details
Furan 2,5-dicarboxylic-acid 3238-40-2 98%View Details
3238-40-2 -
 3238-40-2 Furan-2,5-dicarboxylic acid 98%View Details
3238-40-2 Furan-2,5-dicarboxylic acid 98%View Details
3238-40-2 -
 2,5-Furandicarboxylic acid CAS 3238-40-2View Details
2,5-Furandicarboxylic acid CAS 3238-40-2View Details
3238-40-2 -
 2,5-Furandicarboxylic acid 3238-40-2 98%View Details
2,5-Furandicarboxylic acid 3238-40-2 98%View Details
3238-40-2 -
 2,5-Furandicarboxylic Acid CAS 3238-40-2View Details
2,5-Furandicarboxylic Acid CAS 3238-40-2View Details
3238-40-2 -
 2,5-Furandicarboxylic acid 98%View Details
2,5-Furandicarboxylic acid 98%View Details -
 2,5-Furandicarboxylic acid CAS 3238-40-2View Details
2,5-Furandicarboxylic acid CAS 3238-40-2View Details
3238-40-2 -
 2,5-Furandicarboxylic acid CAS 3238-40-2View Details
2,5-Furandicarboxylic acid CAS 3238-40-2View Details
3238-40-2