Fostamatinib
- CAS NO.:901119-35-5
- Empirical Formula: C23H26FN6O9P
- Molecular Weight: 580.46
- MDL number: MFCD16628163
- EINECS: 618-473-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-21 18:03:03
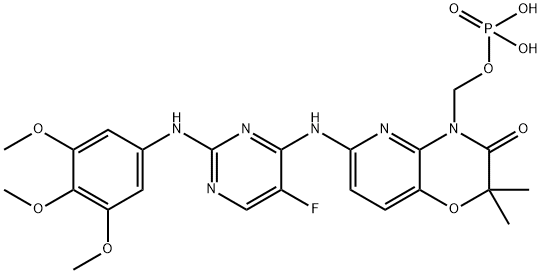
What is Fostamatinib?
Absorption
Fostmatinib is the methylene phosphate prodrug of R406, the active metabolite . It is extensively hydrolyzed by intestinal alkaline phosphatase. Only negligible amounts of fostamatinib enter systemic circulation .
R406 has an absolute bioavailability of 55% and reaches peak plasma concentrations in approximately 1.5 h . Administration with a high calorie, high fat meal increases exposure by 23% and the maximum plasma concentration by 15%. This may lengthen time to peak plasma concentration to approximately 3 h . Exposure to R406 is known to be dose proportional up to 200 mg twice daily . R406 accumulates 2-3 fold with twice daily dosing at 100-160 mg.
Toxicity
Neither fostamatinib or R406 were found to be carcinogenic or mutagenic . Fostamatinib can cause embryo-fetal mortality or developmental abnormalities at exposures of 0.3-10 times the maximum recommended human dose.
Serious adverse effects include hypertension, neutropenia, diarrhea, and hepatotoxicity
The Uses of Fostamatinib
Fostamatinib can be used to treat cancers that have acquired resistance to kinase inhibitors.
Indications
Fostamatinib is indicated for use in the treatment of chronic immune thrombocytopenia (ITP) in patients who have had insufficient response to previous therapy .
What are the applications of Application
R788 is an inhibitor to spleenic kinase-mediated IgG Fc receptor signaling
Background
Fostamatinib has been investigated for the treatment and basic science of Rheumatoid Arthritis and Immune Thrombocytopenic Purpura (ITP). It was approved on April 17, 2018, under the trade name Tavalisse for use in ITP . Fostamatinib has also been granted orphan drug status by the FDA .
Recently, fostamatinib has been identified as a potential therapeutic for controlling acute respiratory distress syndrome (ARDS) in patients with severe COVID-19 through its ability to modulate the SYK kinase.
Indications
Fostamatinib is an FDA-approved first-in-class spleen tyrosine kinase (SYK) inhibitor, specifically indicated for the treatment of adult patients with chronic immune thrombocytopenia (ITP) who have not responded adequately to prior therapies.
brand name
Tavalisse
General Description
Class: non-receptor tyrosine kinase; Treatment: ITP; Other name: R788; Oral bioavailability = 100% (prodrug); Elimination half-life = 15 h (tamatinib); Protein binding = 98.3%
Biological Activity
fostamatinib is a small molecule inhibitor of spleen tyrosine kinase (syk) with ic50 value of 41nm [1].fostamatinib is an orally bioavailable prodrug of r406. it is developed for the treatment of autoimmune diseases. the effective metabolite of fostamatinib, r406, is an atp-competitive inhibitor of syk with ki value of 30nm. r406 also inhibits the activity of other kinases including flt3, lyn (ic50=63nm) and lck (ic50=37nm). it is found that r406 inhibits both bcr and fcr mediated responses in vitro. besides that, r406 also shows effects in other cells types and signalling pathways. in the in vivo assay, fostamatinib shows to be highly active to inhibit fcr-mediated signaling in various animal models of allergy, autoimmunity and inflammation. moreover, fostamatinib also exerts efficacy in sle animal models. treatment of fostamatinib suppresses the established renal and skin disease and reduces lymphadenopathy in the mrl/lpr strain [1].
Pharmacokinetics
The active metabolite of fostamatinib, R406, inhibits signal transduction by Fcγ receptors involved in the antibody-mediated destruction of platelets by immune cells in chronic ITP . This results in increased platelet counts in this population.
R406 produces inhibition of T and B lymphocyte activation by T-cell receptors (TCRs) and B-cell receptors (BCRs) respectively . It can also inhibit signalling via Fcε receptors which could have applications in treating allergic symptoms through prevention of mast cell degranulation . Inhibition of Fc receptor signalling system also affected by R406 suppresses both dendritic cell maturation and antigen presentation and may contribute to the effects of fostamatinib . As a knock-on effect of disabling signal transduction from Fc receptors, TCRs, and BCRs, the production of inflammatory mediators and cytokines like tumour necrosis factor α, leukotriene C4, interleukin-8, and granulocyte-macrophage colony-stimulating factor.
Fostamatinib can produce hypertension through off-target effects
Metabolism
Fostamatinib is metabolized in the gut by alkaline phosphatase to the active metabolite R406 . R406 is further oxidized by CYP3A4 and glucuronidated by UGT1A9. Plasma metabolites found include an O-glucuronide conjugate, an N-glucuronide conjugate, an O-desmethyl metabolite, and a sulfate conjugate . A 3,5 benzene diol metabolite forms in the feces via processing of the O-desmethyl metabolite by gut bacteria .
References
[1] mcadoo s p, tam f w k. fostamatinib disodium. drugs of the future, 2011, 36(4): 273.
Properties of Fostamatinib
| Melting point: | 214° - 217°C |
| Boiling point: | 814.2±75.0 °C(Predicted) |
| Density | 1.496 |
| storage temp. | -20°C, Hygroscopic |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 1.70±0.10(Predicted) |
| color | White to Off-White |
| Stability: | Hygroscopic |
Safety information for Fostamatinib
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Fostamatinib
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid Fmoc-Val-Cit-PAB DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonateRelated products of tetrahydrofuran


You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8