SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 580.5 g/mol |
|---|---|
| XLogP3 | 1.6 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 15 |
| Rotatable Bond Count | 10 |
| Exact Mass | 580.14829159 g/mol |
| Monoisotopic Mass | 580.14829159 g/mol |
| Topological Polar Surface Area | 187 Ų |
| Heavy Atom Count | 40 |
| Formal Charge | 0 |
| Complexity | 904 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
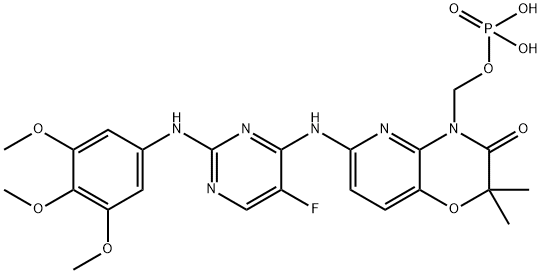
PRODUCT INTRODUCTION
description
Fostamatinib has been investigated for the treatment and basic science of Rheumatoid Arthritis and Immune Thrombocytopenic Purpura (ITP). It was approved on April 17, 2018, under the trade name Tavalisse for use in ITP. Fostamatinib has also been granted orphan drug status by the FDA. Recently, fostamatinib has been identified as a potential therapeutic for controlling acute respiratory distress syndrome (ARDS) in patients with severe COVID-19 through its ability to modulate the SYK kinase.