Foscarnet
- CAS NO.:4428-95-9
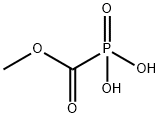
- Empirical Formula: CH3O5P
- Molecular Weight: 126.01
- SAFETY DATA SHEET (SDS)
- Update Date: 2022-12-21 16:56:50
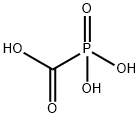
What is Foscarnet?
Absorption
Poorly absorbed after oral administration (bioavailability from 12 to 22%).
Toxicity
Oral, rat LD50: >2,000 mg/kg. Signs of overdose include renal impairment.
Originator
Foscarnet Sodium,AstraZeneca,USA
Indications
For the treatment of CMV retinitis in patients with acquired immunodeficiency syndrome (AIDS) and for treatment of acyclovir-resistant mucocutaneous HSV infections in immunocompromised patients.
Background
An antiviral agent used in the treatment of cytomegalovirus retinitis. Foscarnet also shows activity against human herpes viruses and HIV.
Definition
ChEBI: Phosphoric acid in which one of the hydroxy groups is replaced by a carboxylic acid group. It is used as the trisodium salt as an antiviral agent in the treatment of cytomegalovirus retinitis (CMV retinitis, an inflamation of the retina that can lead to bl ndness) and as an alternative to ganciclovir for AIDS patients who require concurrent antiretroviral therapy but are unable to tolerate ganciclovir due to haematological toxicity.
Manufacturing Process
423 g (4.77 mol) of sodium hydroxide is added to 400 ml of water. The
solution is heated to about 50°C and 167 g (0.795 mol) of triethyl
phosphonoformate is added. The reaction mixture is then heated to about
90°C and ethanol formed is distilled off. After about 1 hour at 90-95°C the
reaction mixture is cooled to about 20°C and the product is filtered off. The
yield of trisodium phosphonoformate hexahydrate wet is 248 g.
The wet substance is recrystallized in 570 ml of water by heating to 423 g
(4.77 mol) of sodium hydroxide liquid conc. is added to 400 ml of water. The
solution is heated to about 50°C and 167 g (0.795 mol) of triethyl
phosphonoformate is added at this temperature. The reaction mixture is then
heated to about 90°C and ethanol formed is distilled off. After about 1 hour at
90-95°C the reaction mixture is cooled to about 20°C and the product is
filtered off. The yield of trisodium phosphonoformate hexahydrate wet is 248
g.
The wet substance is recrystallized in 570 ml of water by heating to 90°C in
order to obtain a clear solution and then cooling to about 20°C. After filtration
and washing with 50 ml of water at 18-22°C 187 g (74% of theoretical yield)
of trisodium phosphonoformate hexahydrate is obtained. This substance
contains about 2% free water, which can be eliminated by drying.
Therapeutic Function
Antiviral
Pharmacokinetics
Foscarnet is an organic analogue of inorganic pyrophosphate that inhibits replication of herpes viruses in vitro including cytomegalovirus (CMV) and herpes simplex virus types 1 and 2 (HSV-1 and HSV-2). Foscarnet does not require activation (phosphorylation) by thymidine kinase or other kinases and therefore is active in vitro against HSV TK deficient mutants and CMV UL97 mutants. Thus, HSV strains resistant to acyclovir or CMV strains resistant to ganciclovir may be sensitive to foscarnet. However, acyclovir or ganciclovir resistant mutants with alterations in the viral DNA polymerase may be resistant to foscarnet and may not respond to therapy with foscarnet. The combination of foscarnet and ganciclovir has been shown to have enhanced activity in vitro.
Enzyme inhibitor
This pyrophosphate analogue (FWtrisodium salt = 191.95 g/mol; CAS 4428-95- 9), also called foscarnet, exhibits antiherpetic activity. It is a stro+ng inhibitor of viral reverse transcriptases and viral replication as well as Na/phosphate cotransporters. Target (s) : adenylyl cyclase; avian myeloblastosis virus reverse transcriptase; cytomegalovirus DNA polymerase (4-6); DNA- directed DNA polymerase; DNA polymerase b; DNA polymerases, viral; duck hepatitis B virus reverse transcriptase ; Epstein-Barr virus DNA polymerase; equine infectious anemia virus reverse transcriptase; exoribonuclease H; feline immunodeficiency virus reverse transcriptase; geranyl-diphosphate cyclase, or bornyl-diphosphate synthase; guanylyl cyclase; HIV-1 reverse transcriptase; human T-lymphotropic virus type III reverse transcriptase; herpes virus reverse transcriptase ; human hepatitis B virus reverse transcripta+se; Moloney murine leukemia virus reverse transcriptase; Na-dependent phosphate transport (32-38); phosphoenolpyruvate carboxylase; phosphoenolpyruvate mutase; phosphonoacetate hydrolase; phosphonopyruvate hydrolase; ribonuclease H; RNA-directed DNA polymerase; simian immunodeficiency virus reverse transcriptase; and woodchuck hepatitis virus reverse transcriptase.
Metabolism
Not metabolized.
Properties of Foscarnet
| Boiling point: | 490.7±28.0 °C(Predicted) |
| Density | 2.142±0.06 g/cm3(Predicted) |
| pka | 0.78±0.10(Predicted) |
Safety information for Foscarnet
Computed Descriptors for Foscarnet
New Products
4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Mefenamic Acid IP/BP/EP/USP Diclofenac Sodium IP/BP/EP/USP Ornidazole IP Diclofenac Potassium SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran


![FOSCARNET SODIUM, [14C]-](https://img.chemicalbook.in/StructureFile/ChemBookStructure4/GIF/CB3748820.gif)
You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4