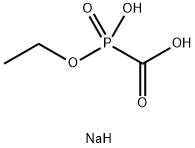
Phosphonoformic acid trisodium salt hexahydrate
Synonym(s):Foscarnet;Phosphonoformic acid trisodium salt hexahydrate
- CAS NO.:34156-56-4
- Empirical Formula: CH6NaO6P
- Molecular Weight: 168.02
- MDL number: MFCD00150176
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-07-02 08:55:06
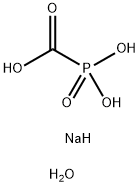
What is Phosphonoformic acid trisodium salt hexahydrate?
Chemical properties
Phosphonoformic acid trisodium salt hexahydrate is white or almost white, crystalline powder.
The Uses of Phosphonoformic acid trisodium salt hexahydrate
Type II Pi transporter inhibitor.
The Uses of Phosphonoformic acid trisodium salt hexahydrate
Foscarnet inhibits viral DNA polymerase and reverse transcriptase. Foscarnet is used as an antiviral.
The Uses of Phosphonoformic acid trisodium salt hexahydrate
Sodium phosphonoformate hexahydrate acts as an antiviral agent and reverse transcriptase inhibitor. It is also used to inhibit viral DNA polymerase. Further, it is used as a type II Pi transporter inhibitor.
What are the applications of Application
Sodium phosphonoformate tribasic hexahydrate is an antiviral agent and reverse transcriptase inhibitor
Definition
ChEBI: The hexahydrate form of trisodium phosphonoformate. It is used as an antiviral agent in the treatment of cytomegalovirus retinitis (CMV retinitis, an inflamation of the retina that can lead to blindness) and as an alternative to ganciclovir for AIDS patien s who require concurrent antiretroviral therapy but are unable to tolerate ganciclovir due to haematological toxicity.
Acquired resistance
Phosphonoformic acid trisodium salt hexahydrate can be generated in vitro, and CMV strains resistant to both ganciclovir and foscarnet have occasionally been recovered from humans.
Pharmaceutical Applications
Phosphonoformic acid trisodium salt hexahydrate is a synthetic non-nucleoside pyrophosphate analog formulated as the trisodium hexahydrate for intravenous use. The solubility in water at pH 7 is only about 5% (w/w).
Pharmacokinetics
Oral absorption: c. 17%
Cmax 60 mg/kg intravenous 8-hourly: 557 μmol/L
Plasma half-life: 3.3–6.8 h
Volume of distribution: 0.52–0.74 L/kg
Plasma protein binding: 14–17%
Absorption and distribution
Oral bioavailability is poor. A wide range of plasma concentrations was noted (75–500 μmol/L) during 3–21 days of continuous intravenous infusion of 0.14–0.19 mg/kg per min. During continuous intravenous therapy the concentrations reached a plateau on day 3. Considerable differences in steady-state plasma concentrations exist between individuals. Drug penetrates the CSF; the mean concentration is about 40–60% of the mean plasma concentration, depending upon dose.
Metabolism and excretion
Elimination appears to be triphasic, with two initially short half-lives of 0.5–1.4 h and 3.3–6.8 h, followed by a long terminal phase of 88 h. About 88% of the cumulative intravenous dose is recovered unchanged in the urine within a week of stopping an infusion, indicating that the drug is not significantly metabolized. Non-renal clearance accounts for 14–18% of total clearance and may relate to uptake into bone. Plasma clearance decreases markedly with decreased renal function and the elimination half-life may be increased by up to 10-fold. Conventional dialysis eliminates about 25% of a dose while high-flux dialysis can remove nearly 60%.
Clinical Use
Treatment of CMV retinitis in patients for whom ganciclovir is
contraindicated, inappropriate or ineffective
It is also potentially of value in the treatment of aciclovir-resistant
HSV infection.
Side Effects
Treatment is more frequently limited by toxicity than with ganciclovir.
Renal toxicity is most common. A two- to three-fold
increase in serum creatinine levels occurs in 20–60% (mean
45%) of patients given 130–230 mg/kg per day as a continuous
intravenous infusion. Renal impairment usually develops
within the first few weeks of treatment and is generally reversible
within several weeks of discontinuing therapy. Foscarnet
chelates metal ions, and serum electrolyte abnormalities –
predominantly hypocalcemia, hypomagnesemia, hypokalemia
and hypophosphatemia – occur in about 30, 15, 16 and 8%
of patients, respectively. Convulsions occur in 10–15%. Other
side effects include anemia (25–50%), penile or vulval ulceration
(3–9%), nausea and vomiting (20–30%), local irritation
and thrombophlebitis at the infusion site, abdominal pain and
occasional pancreatitis, headache (c. 25%), dizziness, involuntary
muscle contractions, tremor, hypoesthesia, ataxia, neuropathy,
anxiety, nervousness, depression and confusion, and
skin rash. Nephrogenic diabetes insipidus has been reported.
Foscarnet is contraindicated in pregnancy. Topical application
does not result in dermal toxicity similar to that produced
by phosphonacetic acid.
Properties of Phosphonoformic acid trisodium salt hexahydrate
| storage temp. | 2-8°C |
| solubility | H2O: 0.1 g/mL hot, clear, colorless |
| form | neat |
| form | Solid |
| color | White to off-white |
| Water Solubility | Soluble in water. |
| CAS DataBase Reference | 34156-56-4(CAS DataBase Reference) |
Safety information for Phosphonoformic acid trisodium salt hexahydrate
| Signal word | Warning |
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H341:Germ cell mutagenicity H373:Specific target organ toxicity, repeated exposure |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P308+P313:IF exposed or concerned: Get medical advice/attention. P405:Store locked up. P501:Dispose of contents/container to..… |
Computed Descriptors for Phosphonoformic acid trisodium salt hexahydrate
| InChIKey | ILRVASBWNRYBFD-UHFFFAOYSA-K |
New Products
Tubulysin M (2S,4R)-4-amino-2-methyl-5-phenylpentanoic acid hydrochloride Tubulysin D Tubulysin C Potassium HMDS (1.0 M in THF) Methyl (R)-1-Boc-4,4-difluoropyrrolidine-2-carboxylate 2H-Pyran, 4-ethynyltetrahydro- 2-(azetidin-3-ylidene)acetonitrile (hydrochloride) 3-N-BOC-(S)-AMINO BUTYRONITRILE Isoxazole, 3-[[[5-(difluoromethoxy)-1-methyl-3-(trifluoromethyl)-1H-pyrazol-4-yl]methyl]thio]-4,5-dihydro-5,5-dimethyl- 4,4-DIFLUOROCYCLOHEXANAMINE (1R,2S)-2-(3,4-Difluorophenyl)cyclopropanamine Fuel shell 2-[2-[3(S)-3[2-(7-chloro-2-quinolinyl) ethenyl] phenyl-3- hydroxyl propyl] phenyl]-2-propanol Imeglimin Hydrochloride IH 2-[[(3aR,4S,6R,6aS)-6-Aminotetrahydro-2,2-dimethyl-4H-cyclopenta-1,3-dioxol-4-yl]oxy]ethanol ethanedioate 2-(1-(Mercaptomethyl) cyclopropyl) acetonitrile Latanoprostene Bunod Magnesium Trisilicate Lubiprostone Flame Retardant Zinc Borate (R)-(3-(3-fluoro-4- thiomorpholinophenyl)-2- oxooxazolidin-4-yl) methyl methanesulfonate methyl 3-fluoro-4- thiomorpholino phenylcarbamate 1H-Imidazole-4-carbonitrileRelated products of tetrahydrofuran
![FOSCARNET SODIUM, [14C]-](https://img.chemicalbook.in/StructureFile/ChemBookStructure4/GIF/CB3748820.gif)


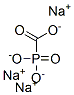

You may like
-
 Phosphonoformic acid trisodium salt hexahydrate 95% CAS 34156-56-4View Details
Phosphonoformic acid trisodium salt hexahydrate 95% CAS 34156-56-4View Details
34156-56-4 -
 Foscarnet sodium CAS 34156-56-4View Details
Foscarnet sodium CAS 34156-56-4View Details
34156-56-4 -
 (R)-(3-(3-fluoro-4- thiomorpholinophenyl)-2- oxooxazolidin-4-yl) methyl methanesulfonateView Details
(R)-(3-(3-fluoro-4- thiomorpholinophenyl)-2- oxooxazolidin-4-yl) methyl methanesulfonateView Details
2416850-45-6 -
 methyl 3-fluoro-4- thiomorpholino phenylcarbamateView Details
methyl 3-fluoro-4- thiomorpholino phenylcarbamateView Details
2760359-22-4 -
 4,6-dichloro-2-propylthiopyrimidine-5-amine 145783-15-9 98%View Details
4,6-dichloro-2-propylthiopyrimidine-5-amine 145783-15-9 98%View Details
145783-15-9 -
 151767-02-1 Montelukast Sodium IP/USP 98%View Details
151767-02-1 Montelukast Sodium IP/USP 98%View Details
151767-02-1 -
 Valacyclovir Hydrochloride IH 98%View Details
Valacyclovir Hydrochloride IH 98%View Details
124832-27-5 -
![2-[2-[3(S)-3[2-(7-chloro-2-quinolinyl) ethenyl] phenyl-3- hydroxyl propyl] phenyl]-2-propanol 98%](https://img.chemicalbook.in//Content/image/CP5.jpg) 2-[2-[3(S)-3[2-(7-chloro-2-quinolinyl) ethenyl] phenyl-3- hydroxyl propyl] phenyl]-2-propanol 98%View Details
2-[2-[3(S)-3[2-(7-chloro-2-quinolinyl) ethenyl] phenyl-3- hydroxyl propyl] phenyl]-2-propanol 98%View Details
142569-70-8