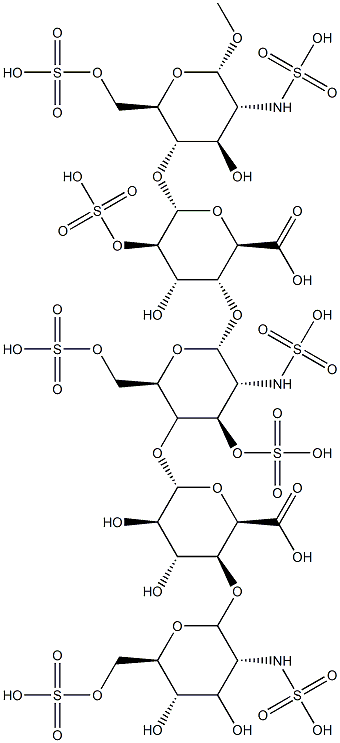
Fondaparinux sodium
Synonym(s):Fondaparin sodium;Fondaparinux sodium;SR-90107A
- CAS NO.:114870-03-0
- Empirical Formula: C31H43N3O49S8.10Na
- Molecular Weight: 1728.08
- MDL number: MFCD06794972
- EINECS: 686-283-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-15 14:23:52
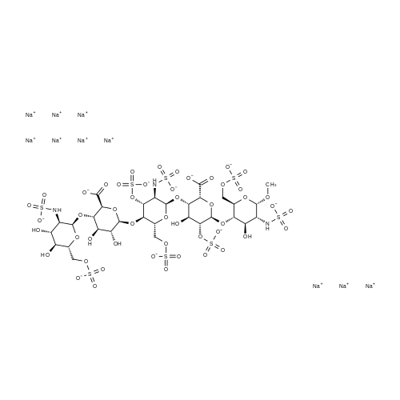
What is Fondaparinux sodium?
Description
Fondaparinux sodium was first introduced in the US for prophylaxis of deep vein thrombosis which may lead to pulmonary embolism following major orthopaedic surgery. Fondaparinux is the first of a new class of antithrombic agents distinct from low molecular weight heparin (LMWH) and heparin. This entirely synthetic molecule is a copy of the heparin pentasaccharide sequence, the shortest fragment able to catalyze antithrombin lllmediated inhibition of factor Xa thereby inhibiting thrombin generation without antithrombin action. Fondaparinux does not display significant effects on coagulation tests (such as activated partial thromboplastin time and prothrombin time), does not bind to platelet factor 4 or promote heparin-induced thrombocytopenia. In phase III studies, fondaparinux significantly reduced the incidence of thromboembolism following orthopedic surgery, with an overall risk reduction of 50% in comparison to the LMWH, enoxaparin. Following subcutaneous administration, fondaparinux has a nearly complete bioavailability, a rapid onset of action, a prolonged half-life (17.2 h) enabling once daily dosing and is not metabolized preceeding renal excretion. The drug appears to be generally safe, with haemoragic complications either comparable to or higher than those for LMWH.
Chemical properties
White Powder (after lyophilisation)
Originator
Sanofi-Synthelabo (France)
The Uses of Fondaparinux sodium
Fondaparinux sodium has been used to test its neutralizing effect towards enterovirus D68-947 infection. It may be used in ultraviolet photodissociation (UVPD) measurements.
The Uses of Fondaparinux sodium
Synthetic pentasaccharide corresponding to the anti-thrombin binding site of heparin. Anti-thrombotic.
Definition
ChEBI: An organic sodium salt, being the decasodium salt of fondaparinux.
Indications
Fondaparinux sodium injection is a Factor Xa inhibitor (anticoagulant) indicated for:
Prophylaxis of deep vein thrombosis (DVT) in patients undergoing hip fracture surgery (including extended prophylaxis), hip replacement surgery, knee replacement surgery, or abdominal surgery.
Treatment of DVT or acute pulmonary embolism (PE) when administered in conjunction with warfarin.
brand name
Arixtra (GlaxoSmithKline).
Biochem/physiol Actions
Fondaparinux sodium is an antithrombotic anticoagulant, a Factor Xa inhibitor. Fondaparinux sodium is chemically related to low molecular weight heparins. Its pentasaccharide structure corresponds to the antithrombin III (ATIII) binding site of heparin. Fondaparinux sodium binding at this site potentiates the natural inhibitory effect of ATIII against factor Xa by a factor of approximately 300, which results in inhibition of thrombin generation.
Clinical Use
Prophylaxis of deep vein thrombosis
Treatment of deep vein thrombosis, pulmonary
embolism, unstable angina and after a myocardial
infarction
Side Effects
Fondaparinux sodium is a prescription medication and as such, most people do not have serious side effects. However, pain, bruising, redness, and swelling at the injection site may occur, as well as headache, nausea, vomiting, swelling of the hands and feet, or fever. A few serious side effects may occur: easy bleeding or bruising; dark urine, yellowing of the eyes or skin; shortness of breath, coughing up blood, chest pain, unusual dizziness, fainting, fast or irregular heartbeat; joint or muscle pain; confusion. Very few patients may experience allergic reactions (e.g. rash, itching or swelling (especially on face/tongue/throat); severe dizziness, difficulty breathing).
Synthesis
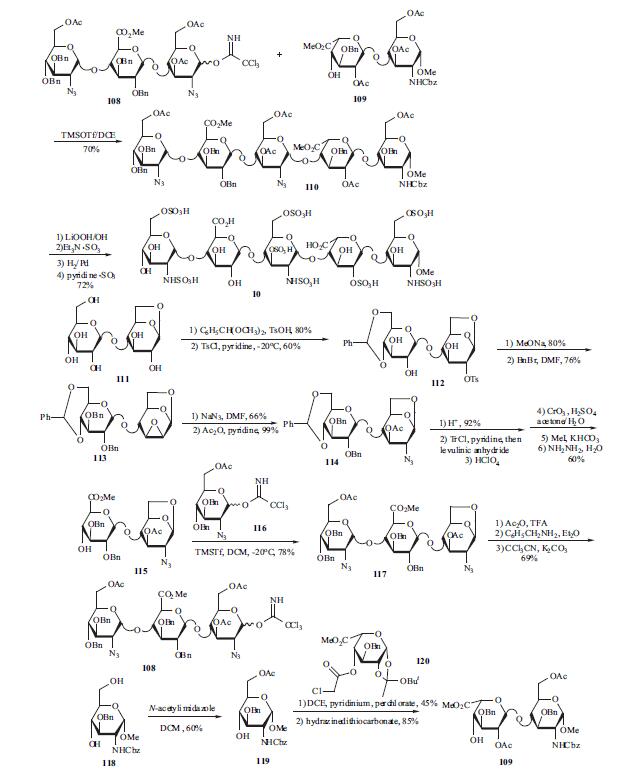
Starting from Dglucose, D-cellobiose, and D-glucosamine, the production process for the synthesis of the pentasaccharide involves about 55 steps. The synthesis was accomplished by preparing a fully-protected pentasaccharide, and then converting it into the final product. The choice of protecting groups was dictated by two factors: the need to introduce sulfate substituents (O- as well as N-linked), carboxylate groups and hydroxyl groups, in the proper positions on the target molecule, and the constraints of current methods for oligosaccharide synthesis, particularly the use of 2-azido glucose derivatives to achieve stereoselective introduction of |á-D-linked glucosamine units. All the monosaccharide synthons were obtained from glucose or from glucosamine, and the synthesis is outlined in the scheme. Trisaccharide 108 and disaccharide 109 are the two key building blocks in the synthesis. Coupling 108 and 109 was carried out at -20??C in DCE. Fully protected pentasaccharide 110 was then converted into the target compound 10 using traditional methods: saponification, O-sulfation, cleavage of benzyl ethers with simultaneous reduction of azido into amino functions and finally N-sulfation. Preparation of trisaccharide building block 108 started from 1,6-anhydrocellobiose (111). Selective protection at 4?ˉ,6?ˉ position was achieved through benzylidenation to provide crude 112 which was converted into epoxide 113 by treatment with sodium methoxide and benzylation. Compound 113 was isolated after filtration on silica gel and crystallization (m.p. 184-5??C). Trans-diaxial opening of the epoxide yielded the 2-azido derivative (66%) which was acetylated to give 114 (99%). The benzylidene was cleaved (92%) and the diol was then converted into 115 by successive tritylation, levulinoylation, detritylation, oxidation, methylation and hydrazinolysis (60% over the 6 steps). Imidate 116 was prepared in the usual way from its hydroxyl precursor and coupled with 115 to give O-linked trisaccharide 117 in 78% yield. Compound 117 was acetolysed (91%), the anomeric acetate was cleaved by benzylamine in ether (100%) and imidate 108 was obtained by reaction with potassium carbonate and trichloroacetonitrile at room temperature (|á, |?- mixture with |á as the predominant isomer, 76%). The preparation of the other building block 109 is described as following. Selective 6-acetylation of 118 by N - acetylimidazole in DCE gave 119 in 60% yield. Treatment of 119 with 120 using DCE/pyridinium perchlorate and followed dechloroacetylation using hydrazinedithiocarbonate afforded the crystalline disaccharide 109.
Drug interactions
Potentially hazardous interactions with other drugs
Increased risk of bleeding in combination with any
other drugs that affect coagulation.
Metabolism
Although not fully evaluated, there is no evidence of fondaparinux metabolism and in particular no evidence for the formation of active metabolites. Fondaparinux is excreted to 64-77% by the kidney as unchanged compound.
Properties of Fondaparinux sodium
| Melting point: | >209°C (dec.) |
| alpha | D23 +48° (c = 0.61 in water) |
| storage temp. | 2-8°C |
| solubility | Water |
| form | Solid |
| color | White to Off-White |
Safety information for Fondaparinux sodium
Computed Descriptors for Fondaparinux sodium
| InChIKey | XEKSTYNIJLDDAZ-UWCYYFDCNA-D |
Fondaparinux sodium manufacturer
Archerchem Healthcare Pvt., Ltd. (part of Archerchem Group)
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
You may like
-
 114870-03-0 Fondaparinux Sodium 98%View Details
114870-03-0 Fondaparinux Sodium 98%View Details
114870-03-0 -
 114870-03-0 98%View Details
114870-03-0 98%View Details
114870-03-0 -
 Fondaparinux sodium 98%View Details
Fondaparinux sodium 98%View Details
114870-03-0 -
 Fondaparinux sodium for Assay CAS 114870-03-0View Details
Fondaparinux sodium for Assay CAS 114870-03-0View Details
114870-03-0 -
 Fondaparinux sodium identification CAS 114870-03-0View Details
Fondaparinux sodium identification CAS 114870-03-0View Details
114870-03-0 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
