Fondaparinux
- CAS NO.:816468-88-9
- Empirical Formula: C31H53N3O49S8
- Molecular Weight: 1508.26322
- MDL number: MFCD00941363
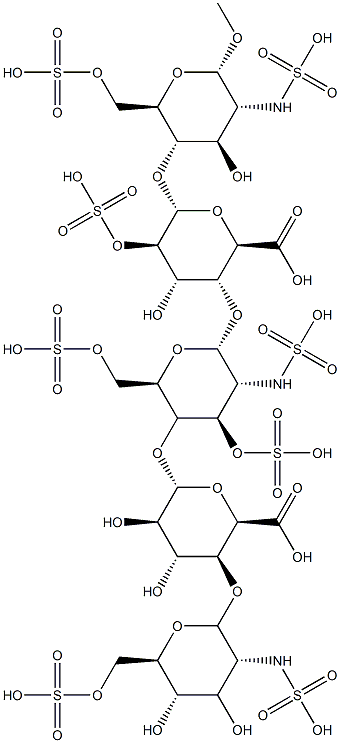
What is Fondaparinux ?
Mechanism of action
The development of fondaparinux, a synthetically derived pentasaccharide that binds specifically to and activates antithrombin III, is a further refinement on the mechanism of action of heparin. Fondaparinus and a related analogue, idraparinux, are specific, indirect inhibitors of activated factor Xa via their activation of antithrombin. Fondaparinux has strategically located sulfonates that bind to antithrombin. Fondaparinux is structurally related to the antithrombotic binding site of heparin. Unlike heparin or LMWHs, however, these inhibitors have no effect on thrombin, because they lack the longer saccharide chains required for binding to thrombin. The highly sulfated heparins exhibit nonselective binding to a number of additional proteins, resulting in decreased bioavailability and significant variation in activity.
Pharmacokinetics
Fondaparinux is administered via subcutaneous injection with a single daily dose and shows complete absorption. The drug is highly bound to antithrombin III (~94%), with no significant binding to other plasma proteins. Because of the predictable anticoagulant effect, the drug does not require routine coagulation monitoring. The drug is not metabolized and is excreted in the urine unchanged within 72 hours in patients with normal renal function. Fondaparinux has an elimination half-life of 17 hours. Presently, no clinically significant drug interactions have been reported.
Clinical Use
Fondaparinux is the first selective factor Xa inhibitor that is approved for the prophylaxis of DVT, which may occur in patients undergoing hip fracture surgery or hip or knee replacement surgery (59).
Side Effects
The most common side effect is major and minor bleeding, and the patient must be carefully monitored. The drug is not to be used when spinal anesthesia or spinal puncture is employed because of the potential for developing a blood clot in the spine. Fondaparinux has not been reported to cause thrombocytopenia, a condition seen with heparin. It is 100% bioavailable, with little or no protein binding.
Safety information for Fondaparinux
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidYou may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4