Fluorochloridone
- CAS NO.:61213-25-0
- Empirical Formula: C12H10Cl2F3NO
- Molecular Weight: 312.12
- MDL number: MFCD00144317
- EINECS: 262-661-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-16 16:15:04
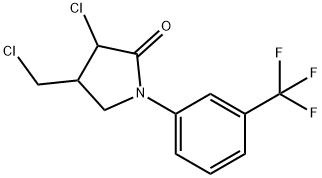
What is Fluorochloridone?
Chemical properties
Brown solid. Easily soluble in acetone, chlorobenzene, ethanol, xylene and other organic solvents, with a solubility of 100-150g/L. Solubility in kerosene <5g/L. The solubility in water is 28mg/L. Partition coefficient (n-octanol/water) 2300 (20°C). The half-life is 7d at 60°C and pH 4, 18d at 60°C and pH 7, and the half-life in soil is 11-100d.
The Uses of Fluorochloridone
Flurochloridone (FLC) is a monophenyl pyrrolidinone selective herbicide that can be used in herbicidal composition to control weed in crop fields. It is commonly used to inhibit weeds that occur during the growth of crop plants, such as grains, sunflowers, and potatoes.
Definition
ChEBI: Flurochloridone is a member of pyrrolidines, a member of (trifluoromethyl)benzenes and an organochlorine compound. It has a role as a carotenoid biosynthesis inhibitor, an agrochemical and a herbicide.
Synthesis
The synthesis of Fluorochloridone is as follows:To a 1L three-neck flask, N-allyl-N-dichloroacetyl-m-trifluoromethylaniline (31.1 g, 0.1 mol) and 715 ml of 1,2-dichloroethane were added as a solvent, dissolved under stirring, and then added. Cuprous chloride (2.97g, 0.03mol) and 2,2-bipyridyl (4.68g, 0.03mol) were reacted at 80°C for 3 hours, cooled to room temperature, and the reaction solution was filtered through a short silica gel column to give dichloromethane. Elution and desolvation yielded 31.3 g of pyrotropone, a content of 97%, an anti-contrast ratio of ≥3:1, and a yield of 97.6%.
Metabolic pathway
From hydrolytic and photolytic studies of flurochloridone in buffer solution, no hydrolysis of flurochloridone occurs at pH 5, 7, or 9 at 25°C or at pH 5 at 40°C. Appreciable hydrolysis occurs at 40°C at pH 7 and 9 with half-lives of 190 and 140 days, respectively. Five hydrolytic degradation products and six photolytic degradation products are identified. The major photolytic degradation product which contains 39% of the radioactivity is 4-(chloromethyl)-3-hydroxy-1-[3- (trifluoromethyl)phenyl]-2-pyrrolidinone with a mixture of cis and trans isomers. The formation of this major degradation product suggests that the major photolytic pathway involves homolytic cleavage of the carbon ? chlorine bond at the 3-position of the pyrrolidinone ring and the free radical intermediate can react with water to give the major product or eliminate the hydrogen radical to form 4- (chloromethyl)-1,5-dihydro-1-[3- (trifluoromethyl)phenyl]pyrrol-2H-one.
Mode of action
Flurochloridone is the active substance of some pre-emergence herbicides. It has an inhibitory effect on the synthesis of carotenoids, which protect chlorophylls against oxidative stress.
Toxicity evaluation
The acute oral LD50 of male rats is 4000 mg/kg, the acute transdermal LD50 of rabbits is >5000 mg/kg, and the acute inhalation of rats is LC50 10.3 mg/L (4h). Slightly irritating to rabbit skin and eyes.
Properties of Fluorochloridone
| Melting point: | 67.65°C |
| Boiling point: | 212.5 °C |
| Density | 1.3924 (estimate) |
| Flash point: | >100 °C |
| storage temp. | under inert gas (nitrogen or Argon) at 2-8°C |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | -3.59±0.60(Predicted) |
| color | Pale Brown to Light Brown |
| CAS DataBase Reference | 61213-25-0(CAS DataBase Reference) |
| NIST Chemistry Reference | 2-Pyrrolidinone, 3-chloro-4-(chloromethyl)-1-[3-(trifluoromethyl)phenyl]-(61213-25-0) |
| EPA Substance Registry System | Flurochloridone (61213-25-0) |
Safety information for Fluorochloridone
Computed Descriptors for Fluorochloridone
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 1-methylindoline-2,3-dione 98%View Details
1-methylindoline-2,3-dione 98%View Details
2058-74-4 -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
