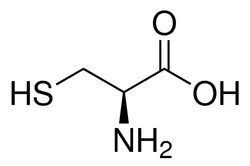
L-Cysteine
Synonym(s):L-Cysteine;Cys;L-Cysteine, Free Base - CAS 52-90-4 - Calbiochem;α-Amino-?-mercapto propionic acid, αlpha-Amino-?-mercapto propionic acid, Cys, αlpha-Amino-beta-mercapto propionic acid;(R)-2-Amino-3-mercaptopropionic acid
- CAS NO.:52-90-4
- Empirical Formula: C3H7NO2S
- Molecular Weight: 121.16
- MDL number: MFCD00064306
- EINECS: 200-158-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 12:07:08
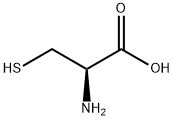
What is L-Cysteine?
Description
Cysteine (abbreviated as Cys or C) is an α-amino acid with the chemical formula HO2CCH(NH2)CH2SH. It is a semi - essential amino acid, which means that it can be biosynthesized in humans. The thiol side chain in cysteine often participates in enzymatic reactions, serving as a nucleophile. The thiol is susceptible to oxidization to give the disulfide derivative cystine, which serves an important structural role in many proteins. When used as a food additive, it has the E number E920.
Description
L-Cysteine is one of the two proteinogenic amino acids that contain a sulfur atom (the other is methionine). It is considered a nonessential amino acid because it is produced in the human body. Individuals who are deficient in cysteine, however, must be treated with supplements.
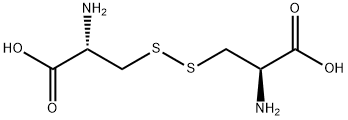
When cysteine is oxidized, the two thiol hydrogen atoms are eliminated, and the sulfur atoms join to form the dimer L-cystine. In biological systems, the monomer and dimer interchange easily; thus, both acids are nutritionally similar.
In September 2018, David Corey at Harvard Medical School and Jeffrey Hold of Boston Children’s Hospital reported that cysteine could play a role in improving hearing in humans. They discovered that the TMC1 protein in the inner ear’s hair cells forms pores that are involved in converting sound to electrical signals to the brain. In some individuals, a mutation in TMC1 can lead to deafness.
The researchers substituted residues in TMC1 with cysteine and then modified the cysteine groups with bulky charged groups. This artificial mutation caused the protein to cease the production of electric current, thus verifying that mutated TMC1 inhibits hearing.
These findings suggest that drugs that target TMC1 mutations or gene therapies that replace the mutated protein could help restore hearing to affected individuals.
Chemical properties
A sulfur-containing amino acid, metabolically related to methionine. Methionine is the source of sulfur atom in the synthesis of cysteine in the body. Chemically, L-cysteine is L-2-amino-mercaptopropionic acid. Cysteine has a sulfureous aroma. It is a nutrient and is used in dietary supplements.
Physical properties
Solubility 28 (25 ℃) g/100 mL solution, 16 (20 ℃) g/100 g H2O, pI 5.02, dissociation constants: pK1 1.71, pK2 8.27 (–SH), pK3 10.78.
Occurrence
Dietary sources
Although classified as a non-essential amino acid, in rare cases, cysteine may be essential for infants, the elderly, and individuals with certain metabolic disease or who suffer from malabsorption syndromes. Cysteine can usually be synthesized by the human body under normal physiological conditions if a sufficient quantity of methionine is available.
Cysteine is catabolized in the gastrointestinal tract and blood plasma. In contrast, cystine travels safely through the GI tract and blood plasma and is promptly reduced to the two cysteine molecules upon cell entry.
Industrial sources
The majority of L - cysteine is obtained industrially by hydrolysis of poultry feathers or human hair. Synthetically produced L-cysteine, compliant with Jewish Kosher and Muslim Halal rules, is also available, albeit at a higher price. The synthetic route involves fermentation utilizing a mutant of E. coli.
Biosynthesis
In animals, biosynthesis begins with the amino acid serine. The sulfur is derived from methionine, which is converted to homocysteine through the intermediate S- adenosylmethionine. Cystathionine betasynthase then combines homocysteine and serine to form the asymmetrical thioether cystathionine. The enzyme cystathionine gamma-lyase converts the cystathionine into cysteine and alphaketobutyrate.
The Uses of L-Cysteine
L-Cysteine is a non-essential amino acid that can be synthesized by the human body under normal physiological conditions if a sufficient quantity of methionine is available. L-Cysteine is commonly used as a precursor in the food and pharmaceutical industries. L-Cysteine is used as a processing aid for baking, as an additive in cigarettes, as well as in the preparation of meat flavours. It is used in foods to prevent oxygen from destroying vitamin c and is used in doughs to reduce mixing time.
Background
A thiol-containing non-essential amino acid that is oxidized to form cystine.
Indications
For the prevention of liver damage and kidney damage associated with overdoses of acetaminophen
What are the applications of Application
L-Cysteine is a non-essential amino acid
Definition
ChEBI: L-cysteine is an optically active form of cysteine having L-configuration. It has a role as a flour treatment agent, a human metabolite and an EC 4.3.1.3 (histidine ammonia-lyase) inhibitor. It is a serine family amino acid, a proteinogenic amino acid, a cysteine and a L-alpha-amino acid. It is a conjugate base of a L-cysteinium. It is a conjugate acid of a L-cysteinate(1-). It is an enantiomer of a D-cysteine. It is a tautomer of a L-cysteine zwitterion.
What are the applications of Application
Cysteine, mainly the L-enantiomer, is a precursor in the food, pharmaceutical, and personal care industries. One of the largest applications is the production of flavors. For example, the reaction of cysteine with sugars in a Maillard reaction yields meat flavors. Lcysteine is also used as a processing aid for baking.
In the field of personal care, cysteine is used for permanent wave applications predominantly in Asia. Again the cysteine is used for breaking up the disulfide bonds in the hair's keratin.
Cysteine is a very popular target for site-directed labeling experiments to investigate biomolecular structure and dynamics. Maleimides will selectively attach to cysteine using a covalent Michael addition. Site- directed spin labeling for EPR or paramagnetic relaxation enhanced NMR also uses cysteine extensively.
cysteine is an essential amino acid obtained by fermentation. Cysteine is a component of the skin’s natural moisturizing factor and can help normalize oil gland secretion because of its sulfur content. It is also said to promote wound healing. In addition, studies indicate that cysteine helps increase levels of glutathione (an anti-oxidant) in the body. It is considered beneficial in treating oily skin.
Preparation
L-Cysteine used to be produced almost exclusively by hydrolysis of hair or other keratins. The amino acid isolated was l-cystine, which was reduced electrolytically to l-cysteine. L-Cysteine has also been prepared from beta-chloro-d,l-alanine and sodium sulfide with cysteine desulfhydrase, an enzyme obtained from, e.g., Citrobacterium freundii. Today, however, the main processes for cysteine production are biological. A direct fermentation process has been developed for the manufacture of l-cystine, using a modified Escherichia coli bacterium. The technology has been extended to prepare other modified l-cysteine analogues. An enzymatic process for l-cysteine has been successfully developed using microorganisms capable to hydrolyze 2-amino-delta2-thiazoline 4-carboxylic acid (ATC) which is readily available from methyl alpha-chloroacrylate and thiourea. A mutant of Pseudomonas thiazolinophilum converts d,l-ATC to l-cysteine in 95% molar yield at product concentrations higher than 30 g/L.
Biological Functions
The cysteine thiol group is nucleophilic and easily oxidized. The reactivity is enhanced when the thiol is ionized, and cysteine residues in proteins have pKa values close to neutrality, so are often in their reactive thiolate form in the cell. Because of its high reactivity, the thiol group of cysteine has numerous biological functions.
Precursor to the antioxidant glutathione
Due to the ability of thiols to undergo redox reactions, cysteine has antioxidant properties. Cysteine's antioxidant properties are typically expressed in the tripeptide glutathione, which occurs in humans as well as other organisms.
Precursor to iron-sulfur clusters
Cysteine is an important source of sulfide in human metabolism. The sulfide in iron-sulfur clusters and in nitrogenase is extracted from cysteine, which is converted to alanine in the process.
Metal ion binding
Beyond the iron - sulfur proteins, many other metal cofactors in enzymes are bound to the thiolate substituent of cysteinyl residues. Examples include zinc in zinc fingers and alcohol dehydrogenase, copper in the blue copper proteins, iron in cytochrome P450, and nickel in the [NiFe]-hydrogenases . The thiol group also has a high affinity for heavy metals, so that proteins containing cysteine, such as metallothionein, will bind metals such as mercury, lead, and cadmium tightly.
Roles in protein structure
In the translation of messenger RNA molecules to produce polypeptides, cysteine is coded for by the UGU and UGC codons. Cysteine has traditionally been considered to be a hydrophilic amino acid, based largely on the chemical parallel between its thiol group and the hydroxyl groups in the side-chains of other polar amino acids. However, the cysteine side chain has been shown to stabilize hydrophobic interactions in micelles to a greater degree than the side chain in the non-polar amino acid glycine, and the polar amino acid serine .
Synthesis Reference(s)
Chemical and Pharmaceutical Bulletin, 34, p. 869, 1986 DOI: 10.1248/cpb.34.869
General Description
L-cysteine is a sulfur-containing non-essential amino acid. Its ability to reduce colitis symptoms is being assessed for potential use in treating inflammatory bowel disease (IBD).
Biochem/physiol Actions
NMDA glutamatergic receptor agonist.
Pharmacokinetics
Due to this ability to undergo redox reactions, cysteine has antioxidant properties. Cysteine is an important source of sulfur in human metabolism, and although it is classified as a non-essential amino acid, cysteine may be essential for infants, the elderly, and individuals with certain metabolic disease or who suffer from malabsorption syndromes. Cysteine may at some point be recognized as an essential or conditionally essential amino acid.
Side Effects
Cysteine has been proposed as a preventative or antidote for some of the negative effects of alcohol, including liver damage and hangover. It counteracts the poisonous effects of acetaldehyde, which is the major by - product of alcohol metabolism and is responsible for most of the negative aftereffects and long - term damage associated with alcohol use (but not the immediate effects of drunkenness). Cysteine supports the next step in metabolism, which turns acetaldehyde into the relatively harmless acetic acid. In a rat study, test animals received an LD50 dose of acetaldehyde. Those that received cysteine had an 80 % survival rate; when both cysteine and thiamine were administered, all animals survived . There is not yet direct evidence for or against its effectiveness in humans who consume alcohol at normal levels.
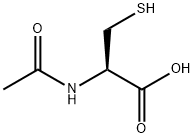
N-Acetylcysteine
N - Acetyl - L - cysteine (NAC) is a derivative of cysteine wherein an acetyl group is attached to the nitrogen atom. This compound is sold as a dietary supplement and used as an antidote in cases of acetaminophen overdose, and obsessive compulsive disorders such as trichotillomania.
Safety Profile
Moderately toxic by ingestion, intraperitoneal, and subcutaneous routes. Experimental reproductive effects. Human mutation data reported. When heated to decomposition fumes of SO and NO.
Metabolism
Not Available
Purification Methods
Purify it by recrystallisation from H2O (free from metal ions) and dry it in a vacuum. It is soluble in H2O, EtOH, Me2CO, EtOAc, AcOH, *C6H6 and CS2. Acidic solutions can be stored under N2 for a few days without deterioration. [For synthesis and spectra see Greenstein & Winitz Chemistry of the Amino Acids (J. Wiley) Vol 3 p1879 1961, Beilstein 4 III 1618, 4 IV 3144.]
Sheep
Cysteine is required by sheep in order to produce wool: It is an essential amino acid that must be taken in as food from grass. As a consequence, during drought conditions, sheep stop producing wool; however, transgenic sheep that can make their own cysteine have been developed.
Properties of L-Cysteine
| Melting point: | 240 °C (dec.) (lit.) |
| Boiling point: | 293.9±35.0 °C(Predicted) |
| alpha | 8.75 º (c=12, 2N HCl) |
| Density | 1.197 (estimate) |
| refractive index | 8.8 ° (C=8, 1mol/L HCl) |
| FEMA | 3263 | L-CYSTEINE |
| storage temp. | Store below +30°C. |
| solubility | H2O: 25 mg/mL |
| appearance | white crystals or powder |
| form | Solid |
| pka | 1.92(at 25℃) |
| color | White |
| PH | 4.5-5.5 (100g/l, H2O, 20℃) |
| Odor | sulfury |
| optical activity | Optical rotation: +8° to +9° (c = 5, 1 N HCl, 20°C). |
| Water Solubility | 280 g/L (25 ºC) |
| Sensitive | Air Sensitive |
| λmax | λ: 260 nm Amax: 1.5 λ: 280 nm Amax: 0.2 |
| JECFA Number | 1419 |
| Merck | 14,2781 |
| BRN | 1721408 |
| Stability: | Stability Stable, but may be air sensitive. Incompatible with oxidizing agents, bases. |
| CAS DataBase Reference | 52-90-4(CAS DataBase Reference) |
| NIST Chemistry Reference | L-Cysteine(52-90-4) |
| EPA Substance Registry System | L-Cysteine (52-90-4) |
Safety information for L-Cysteine
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P330:Rinse mouth. |
Computed Descriptors for L-Cysteine
L-Cysteine manufacturer
Clickchem Research LLP
Aditya Molecules
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 L-Cysteine 99% supplierView Details
L-Cysteine 99% supplierView Details
52-90-4 -
 L-Cysteine 99%View Details
L-Cysteine 99%View Details -
 L-Cysteine extrapure CHR CAS 52-90-4View Details
L-Cysteine extrapure CHR CAS 52-90-4View Details
52-90-4 -
 L-Cysteine CASView Details
L-Cysteine CASView Details -
 L-Cysteine 99% CAS 52-90-4View Details
L-Cysteine 99% CAS 52-90-4View Details
52-90-4 -
 CysteineView Details
CysteineView Details
52-90-4 -
 Food & Pharmaceutical L-cysteine (cas no. 52-90-4), Packaging Type: Drum, Packaging Size: 25kgView Details
Food & Pharmaceutical L-cysteine (cas no. 52-90-4), Packaging Type: Drum, Packaging Size: 25kgView Details
52-90-4 -
 Tetrahydrofuran, 99%View Details
Tetrahydrofuran, 99%View Details
52-90-4
