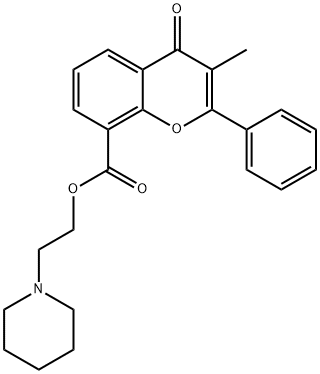
FLAVOXATE
- CAS NO.:15301-69-6
- Empirical Formula: C24H25NO4
- Molecular Weight: 391.46
- MDL number: MFCD00210291
- EINECS: 239-337-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-04-23 13:52:06
What is FLAVOXATE?
Absorption
Well absorbed from gastrointestinal tract.
Toxicity
The oral LD50 for flavoxate HCl in rats is 4273 mg/kg. The oral LD50 for flavoxate HCl in mice is 1837 mg/kg. Symptoms of overdose include convulsions, decreased ability to sweat, (warm, red skin, dry mouth, and increased body temperature), hallucinations, increased heart rate and blood pressure, and mental confusion.
Originator
Urispas,SKF,US,1971
The Uses of FLAVOXATE
Flavoxate is a pharmaceutical active-containing film delivery device for oral transmucosal administration.
The Uses of FLAVOXATE
Relaxant (smooth muscle).
Background
A drug that has been used in various urinary syndromes and as an antispasmodic. Its therapeutic usefulness and its mechanism of action are not clear. It may have local anesthetic activity and direct relaxing effects on smooth muscle as well as some activity as a muscarinic antagonist. [PubChem]
Indications
For symptomatic relief of dysuria, urgency, nocturia, suprapubic pain, frequency and incontinence as may occur in cystitis, prostatitis, urethritis, urethrocystitis/urethrotrigonitis.
What are the applications of Application
BML-278 is a Sirt activator and cell cycle arresting compound
Definition
ChEBI: A carboxylic ester resulting from the formal condensation of 3-methylflavone-8-carboxylic acid with 2-(1-piperidinyl)ethanol.
Manufacturing Process
A mixture of 13.3 grams of anhydrous aluminum chloride and 100 ml of
carbon disulfide is added to 19.4 grams of 2-propionyloxybenzoic acid
(prepared from the reaction of propionyl chloride and 2-hydroxybenzoic acid).
After an initial evolution of hydrogen chloride, the solvent is removed by
distillation and the mixture is heated at 150° to 160°C for 4 hours. The cooled
reaction mixture is treated with ice and hydrochloric acid and the product, 2-
hydroxy-3-carboxypropiophenone, is obtained from the oily residue by
distillation in vacuo.
A mixture of 1.9 grams of 2-hydroxy-3-carboxypropiophenone, 5.0 grams of
sodium benzoate and 20.0 grams of benzoic anhydride is heated at 180° to
190°C for 6 hours. A solution of 15.0 grams of potassium hydroxide in 50 ml
of ethanol and 20 ml of water is added and refluxed for 1 hour. The mixture is
evaporated and the residue after addition of water yields 3-methylflavone-8-
carboxylic acid.
To a suspension of 12.0 grams of 3-methylflavone-8-carboxylic acid in 200 ml
of anhydrous benzene is added 10.0 grams of thionyl chloride. The mixture is
refluxed for 2 hours during which the suspended solid goes into solution. The
solvent is completely removed by distillation, the residue extracted with
benzene and the extract evaporated to dryness. The product, 3-
methylflavone-8-carboxylic acid chloride, is recrystallized from ligroin to give
crystals melting at 155° to 156°C.
To 11.0 grams of 3-methylflavone-8-carboxylic acid chloride dissolved in 150
ml of anhydrous benzene is added at room temperature 4.8 grams of
piperidinoethanol and the mixture refluxed for 2 to 3 hours. The separated
solid is filtered, washed with benzene and dried. The product, piperidinoethyl
3-methylflavone-8-carboxylate hydrochloride is obtained as a colorless
crystalline solid, MP 232° to 234°C, (from US Patent 2,921,070).
brand name
Urispas (Ortho-McNeil).
Therapeutic Function
Spasmolytic
Pharmacokinetics
Flavoxate is a spasmolytic flavone derivative that acts by relaxing the smooth muscle in the urinary tract. Flavoxate is a competitive muscarinic receptor antagonist indicated for the treatment of overactive bladder with symptoms of urge urinary incontinence, urgency, and urinary frequency. Muscarinic receptors play an important role in several major cholin-ergically mediated functions, including contractions of urinary bladder smooth muscle and stimulation of salivary secretion.
Metabolism
Not Available
Properties of FLAVOXATE
| Melting point: | 87-88 °C |
| Boiling point: | 564.1±50.0 °C(Predicted) |
| Density | 1.203±0.06 g/cm3(Predicted) |
| form | Off-white solid. |
| pka | 7.3(at 25℃) |
| CAS DataBase Reference | 15301-69-6(CAS DataBase Reference) |
Safety information for FLAVOXATE
Computed Descriptors for FLAVOXATE
FLAVOXATE manufacturer
New Products
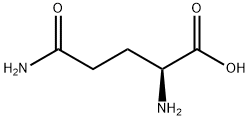
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran

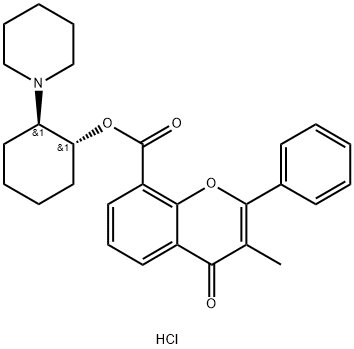
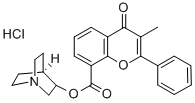
You may like
-
 15301-69-6 Flavoxate 99%View Details
15301-69-6 Flavoxate 99%View Details
15301-69-6 -
 17604-74-9 3-Pyridineacetonitrile, α-hydroxy- 98+View Details
17604-74-9 3-Pyridineacetonitrile, α-hydroxy- 98+View Details
17604-74-9 -
 131987-69-4 98+View Details
131987-69-4 98+View Details
131987-69-4 -
 Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
67967-07-1 -
 764-60-3 2-Hexyn-1-ol 98+View Details
764-60-3 2-Hexyn-1-ol 98+View Details
764-60-3 -
 2-Propanamine, 1-chloro-, hydrochloride (9CI) 98+View Details
2-Propanamine, 1-chloro-, hydrochloride (9CI) 98+View Details
5968-21-8 -
 3-Iodophenylacetic acid 1878-69-9 98+View Details
3-Iodophenylacetic acid 1878-69-9 98+View Details
1878-69-9 -
 132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6
