Vincamine
- CAS NO.:1617-90-9
- Empirical Formula: C21H26N2O3
- Molecular Weight: 354.44
- MDL number: MFCD00078054
- EINECS: 216-576-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 12:07:08
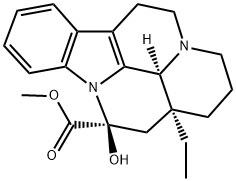
What is Vincamine?
Chemical properties
white to almost white fine crystalline powder
Originator
Pervancamine ,Dausse,France,1969
The Uses of Vincamine
Vincamine is often used as a nootropic agent to combat the effects of aging, or in conjunction with other nootropics (such as piracetam) for a variety of purposes. Vincamine is a peripheral vasodilator that increases blood flow to the brain.
What are the applications of Application
Vincamine is a peripheral vasodilator
Definition
ChEBI: Vincamine is a vinca alkaloid, an alkaloid ester, an organic heteropentacyclic compound, a methyl ester and a hemiaminal. It has a role as an antihypertensive agent, a vasodilator agent and a metabolite. It is functionally related to an eburnamenine.
Manufacturing Process
The following route is described in US Patent 4,145,552: At ambient temperature, over a period of thirty minutes, a solution of 33.8g (0.1mol) of (-)-vincadiformine in a mixture of 140 ml of anhydrous dimethylformamide and 140 ml of anhydrous toluene is added to a suspension of 2.64 g (0.11 mol) of sodium hydride in a mixture of 200 ml of anhydrous tetrahydrofuran, 20 ml of anhydrous hexamethylphosphotriamide (EMPT) and 18.7 ml (0.14 mol) of trimethyl phosphite. When the release of hydrogen has finished (about two hours later), the solution is cooled to -10°C and then stirred under an oxygen atmosphere until absorption ceases (duration: 3 hours). Still at -10°C, 136 ml of glacial acetic acid are added, and the mixture is then left at ambient temperature for two hours. After the addition of 500 ml of 1 N sulfuric acid, the aqueous phase is isolated, reextracted with 150 ml of isopropyl ether, made alkaline with 350 ml of 11 N ammonia, then extracted 3 times with 300 ml aliquots of methylene chloride. After drying over calcium chloride and evaporating the solvent, 30.2 g of crude product are obtained which, when chromatographed on a column of silica gel (1.5 kg) yield, 9.9 g of vincamine (yield: 28%) melting point (decomp.): 250°C.
brand name
Cerebroxine;Cetal;Ocu-vinc;Oxygeron;Pervincamine;Vadicate;Vinca minor;Vincacen;Vincapront;Vincavix;Vincimax.
Therapeutic Function
Vasodilator
World Health Organization (WHO)
Vincamine, an alkaloid derived from Vinca minor, is claimed to increase cerebral circulation and utilization of oxygen. It is used in a variety of cerebral disorders and is widely marketed for this purpose.
General Description
Vincamine is a monoterpenoid indole alkaloid found in the leaves of Vinca minor L., belonging to the Apocynaceae family.
Properties of Vincamine
| Melting point: | 232 °C (dec.)(lit.) |
| alpha | 42.8 º (c=1 in pyridine) |
| Boiling point: | 487.66°C (rough estimate) |
| Density | 1.1640 (rough estimate) |
| refractive index | 1.6500 (estimate) |
| storage temp. | Keep in dark place,Inert atmosphere,2-8°C |
| solubility | Chloroform (Slightly), DMSO (Slightly) |
| form | Solid |
| pka | 12.13±0.40(Predicted) |
| color | White to Off-White |
| optical activity | [α]23/D +42.8°, c = 1 in pyridine |
| Merck | 14,9983 |
| Stability: | Hygroscopic |
| NIST Chemistry Reference | Vincamine(1617-90-9) |
Safety information for Vincamine
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P501:Dispose of contents/container to..… |
Computed Descriptors for Vincamine
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran







You may like
-
 1617-90-9 Vincamine 99%View Details
1617-90-9 Vincamine 99%View Details
1617-90-9 -
 Vincamine CAS 1617-90-9View Details
Vincamine CAS 1617-90-9View Details
1617-90-9 -
 Vincamine 98% (HPLC) CAS 1617-90-9View Details
Vincamine 98% (HPLC) CAS 1617-90-9View Details
1617-90-9 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
