Pentoxifylline
Synonym(s):3,7-Dimethyl-1-(5-oxohexyl)xanthine;Trental
- CAS NO.:6493-05-6
- Empirical Formula: C13H18N4O3
- Molecular Weight: 278.31
- MDL number: MFCD00063379
- EINECS: 229-374-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 13:58:55
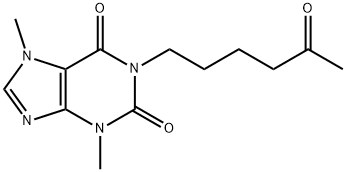
What is Pentoxifylline?
Absorption
Oral pentoxifylline (PTX) is almost completely absorbed but has low bioavailability of 20-30% due to extensive first-pass metabolism; three of the seven known metabolites, M1, M4, and M5 are present in plasma and appear soon after dosing. Single oral doses of 100, 200, and 400 mg of pentoxifylline in healthy males produced a mean tmax of 0.29-0.41 h, a mean Cmax of 272-1607 ng/mL, and a mean AUC0-∞ of 193-1229 ng*h/mL; corresponding ranges for metabolites 1, 4, and 5 were 0.72-1.15, 114-2753, and 189-7057. Single administration of a 400 mg extended-release tablet resulted in a heightened tmax of 2.08 ± 1.16 h, lowered Cmax of 55.33 ± 22.04 ng/mL, and a comparable AUC0-t of 516 ± 165 ng*h/mL; all these parameters were increased in cirrhotic patients.
Smoking was associated with a decrease in the Cmax and AUCsteady-state of metabolite M1 but did not dramatically affect the pharmacokinetic parameters of pentoxifylline or other measured metabolites. Renal impairment increases the mean Cmax, AUC, and ratio to parent compound AUC of metabolites M4 and M5, but has no significant effect on PTX or M1 pharmacokinetics. Finally, similar to cirrhotic patients, the Cmax and tmax of PTX and its metabolites are increased in patients with varying degrees of chronic heart failure.
Overall, metabolites M1 and M5 exhibit plasma concentrations roughly five and eight times greater than PTX, respectively. PTX and M1 pharmacokinetics are approximately dose-dependent, while those of M5 are not. Food intake before PTX ingestion delays time to peak plasma concentrations but not overall absorption. Extended-release forms of PTX extend the tmax to between two and four hours but also serves to ameliorate peaks and troughs in plasma concentration over time.
Toxicity
Overdoses of pentoxifylline have been reported with symptoms including agitation, fever, flushing, hypotension, convulsions, somnolence, and loss of consciousness beginning 4-5 hours following ingestion and lasting up to 12 hours. Symptomatic treatment is recommended, specifically pertaining to maintaining proper respiration, blood pressure, and controlling convulsions. Activated charcoal may prove useful in absorbing excess pentoxifylline in overdose cases. Patients have recovered from overdose even at doses as high as 80 mg/kg.
Chemical properties
White to Off-White Solid
Originator
Trental,Albert Roussel ,W. Germany ,1972
The Uses of Pentoxifylline
Pentoxifylline is a methylxanthine phospho-diesterase inhibitor with favorable antiinflammatory effects and immunoregulatory properties.
A metabolite of Pentifylline. Methylxanthine derivative that improves blood flow by decreasing blood viscosity. Phosphodiesterase inhibitor. Inhibits the synthesis of tumor necrosis factor a (TNF-a).
The Uses of Pentoxifylline
Pentoxifylline can increase red blood cell deformability, reduce blood viscosity, and decrease platelet aggregation and thrombus formation. Pentoxifylline is an oral agent that improves perfusion of occluded vessels and is used to treat systemic vascular diseases. The retinal flow velocity in patients with RVO treated with pentoxifylline improved compared with placebo, but the study had a small number of patients (n = 8) and short follow-up (4 weeks), and no visual outcome data were reported.
The Uses of Pentoxifylline
Pentoxifylline has been used:
- used in the combinatorial treatment with itraconazole for paracoccidioidomycosis (PCM)
- to treat harvested sperms to check the effect of ′pentoxifylline exposed sperms′ in the contribution of embryonic growth
- to intrathecally inject female and male mice to investigate whether astrocytes and microglia could be causally involved in the maintenance of pain-like behaviour
Background
Pentoxifylline (PTX) is a synthetic dimethylxanthine derivative that modulates the rheological properties of blood and also has both anti-oxidant and anti-inflammatory properties. Although originally developed to treat intermittent claudication, a form of exertion-induced leg pain common in patients with peripheral arterial disease, PTX has been investigated for its possible use in diverse conditions, including osteoradionecrosis, diabetic kidney disease, and generally any condition associated with fibrosis. More recently, PTX has been suggested as a possible treatment for COVID-19-induced pulmonary complications due to its ability to regulate the production of inflammatory cytokines.
Pentoxifylline has been marketed in Europe since 1972; PTX extended-release tablets sold under the trade name TRENTAL by US Pharm Holdings were first approved by the FDA on Aug 30, 1984, but have since been discontinued. A branded product, PENTOXIL, marketed by Upsher-Smith Laboratories, and generic forms marketed by Valeant Pharmaceuticals and APOTEX have been available since the late 1990s.
Indications
Pentoxifylline is indicated for the treatment of intermittent claudication in patients with chronic occlusive arterial disease. Pentoxifylline may improve limb function and reduce symptoms but cannot replace other therapies such as surgical bypass or removal of vascular obstructions.
What are the applications of Application
Pentoxifylline is a non-selective phosphodiesterase inhibitor. Inhibits endotoxin-induced synthesis of TNF-α
Indications
Pentoxifylline at a dose of 400 mg three times a day after meals was reported to alleviate the symptoms in a small open trial.
Definition
ChEBI: Pentoxifylline is an oxopurine.
Manufacturing Process
A solution of 35.4 g of 1-bromohexanone-5 in 200 ml of ethanol was gradually mixed at the reflux temperature with vigorous stirring with 39.7 g of theobromine-sodium in 100 ml of water. After 3 hours reflux the unreacted theobromine was filtered off with suction, the filtrate was evaporated to dryness, the residue was dissolved in water and the solution was extracted with chloroform. The chloroform was distilled off and 1-(5'-oxohexyl)-3,7 Pentoxifylline dimethylxanthine was obtained as residue; after recrystallization from isopropanol, it melted at 102°C to 103°C (about 25% yield, calculated on the reacted theobromine).
brand name
Pentoxil (Upsher Smith); Trental (Sanofi Aventis).
Therapeutic Function
Vasodilator
Biological Activity
Phosphodiesterase inhibitor that blocks production of TNF- α and other cytokines. Displays antinociceptive activity.
Biochem/physiol Actions
Pentoxifylline?(PTX) is considered as a nonspecific phosphodiesterase inhibitor. It possesses rheologic properties. Pentoxifylline?is used to treat peripheral vascular disease. It has the ability to block the phosphorylation of I kappa B-alpha (I?Bα) in serines 32 and 36.
Pharmacokinetics
Pentoxifylline, a synthetic dimethylxanthine derivative structurally related to theophylline and caffeine, exhibits hemorheological, anti-oxidative, and anti-inflammatory properties and is traditionally indicated in the treatment of peripheral arterial disease (PAD). In PAD patients with concurrent cerebrovascular and coronary artery diseases, pentoxifylline treatment has occasionally been associated with angina, arrhythmia, and hypotension. Concurrent use with warfarin should be associated with more frequent monitoring of prothrombin times. Also, patients with risk factors complicated by hemorrhages, such as retinal bleeding, peptic ulceration, and recent surgery, should be monitored periodically for bleeding signs.
Veterinary Drugs and Treatments
In horses, pentoxifylline has been used as adjunctive therapy for cutaneous,
vasculitis, endotoxemia and for the treatment of navicular
disease.
Pentoxifylline has been used in dogs to treat immune-mediated
dermatologic conditions, enhance healing, and reduce inflammation
caused by ulcerative dermatosis in Shelties and Collies and
for other conditions where improved microcirculation may be of
benefit. It is being investigated for adjunctive therapy for dilated
cardiomyopathy in Doberman pinschers.
Pentoxifylline has been tried in conjunction with prednisolone
to decrease vasculitis associated with FIP in cats.
Pentoxifylline’s major indications for humans include symptomatic
treatment of peripheral vascular disease (e.g., intermittent claudication,
sickle cell disease, Raynaud’s, etc.) and cerebrovascular diseases
where blood flow may be impaired in the microvasculature.
Metabolism
Pentoxifylline (PTX) metabolism is incompletely understood. There are seven known metabolites (M1 through M7), although only M1, M4, and M5 are detected in plasma at appreciable levels, following the general pattern M5 > M1 > PTX > M4. As PTX apparent clearance is higher than hepatic blood flow and the AUC ratio of M1 to PTX is not appreciably different in cirrhotic patients, it is clear that erythrocytes are the main site of PTX-M1 interconversion. However, the reaction likely occurs in the liver as well. PTX is reduced in an NADPH-dependent manner by unknown an unidentified carbonyl reductase to form either lisofylline (the (R)-M1 enantiomer) or (S)-M1; the reaction is stereoselective, producing (S)-M1 exclusively in liver cytosol, 85% (S)-M1 in liver microsomes, and a ratio of 0.010-0.025 R:S-M1 after IV or oral dosing in humans. Although both (R)- and (S)-M1 can be oxidized back into PTX, (R)-M1 can also give rise to M2 and M3 in liver microsomes. In vitro studies suggest that CYP1A2 is at least partly responsible for the conversion of lisofylline ((R)-M1) back into PTX. Unlike the reversible oxidation/reduction of PTX and its M1 metabolites, M4 and M5 are formed via irreversible oxidation of PTX in the liver. Studies in mice recapitulating the PTX-ciprofloxacin drug reaction suggest that CYP1A2 is responsible for the formation of M6 from PTX and of M7 from M1, both through de-methylation at position 7. In general, metabolites M2, M3, and M6 are formed at very low levels in mammals.
Properties of Pentoxifylline
| Melting point: | 98-100°C |
| Boiling point: | 421.13°C (rough estimate) |
| Density | 1.1713 (rough estimate) |
| refractive index | 1.6000 (estimate) |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | H2O: ≥43 mg/mL |
| form | solid |
| pka | 0.50±0.70(Predicted) |
| color | white |
| λmax | 276nm(lit.) |
| Merck | 14,7136 |
| CAS DataBase Reference | 6493-05-6(CAS DataBase Reference) |
| NIST Chemistry Reference | Pentoxifylline(6493-05-6) |
| EPA Substance Registry System | 1H-Purine-2,6-dione, 3,7-dihydro-3,7-dimethyl-1-(5-oxohexyl)- (6493-05-6) |
Safety information for Pentoxifylline
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. |
Computed Descriptors for Pentoxifylline
Pentoxifylline manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
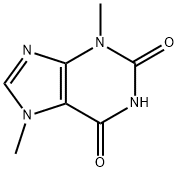
![3,7-Dihydro-3,7-diMethyl-6-[(5-oxohexyl)oxy]-2H-purin-2-one](https://img.chemicalbook.in/CAS/GIF/93079-86-8.gif)
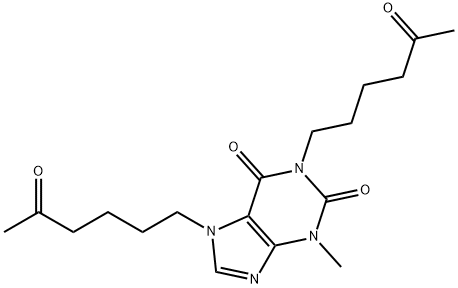
![1,1'-[(5E)-5-Methyl-7-oxo-5-undecene-1,11-diyl] Bis](https://img.chemicalbook.in/CAS/GIF/874747-30-5.gif)
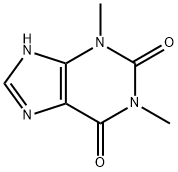
![1,1'-Methylene Bis[TheobroMine]](https://img.chemicalbook.in/CAS/GIF/77196-87-3.gif)

You may like
-
 6493-05-6 Pentoxifylline 98%View Details
6493-05-6 Pentoxifylline 98%View Details
6493-05-6 -
 Pentoxifylline 98%View Details
Pentoxifylline 98%View Details
6493-05-6 -
 Pentoxifylline 6493-05-6 99%View Details
Pentoxifylline 6493-05-6 99%View Details
6493-05-6 -
 Pentoxifylline 99%View Details
Pentoxifylline 99%View Details
6493-05-6 -
 Pentoxifylline 6493-05-6 98%View Details
Pentoxifylline 6493-05-6 98%View Details
6493-05-6 -
 6493-05-6 Pentoxifylline 99%View Details
6493-05-6 Pentoxifylline 99%View Details
6493-05-6 -
 Pentoxifylline CAS 6493-05-6View Details
Pentoxifylline CAS 6493-05-6View Details
6493-05-6 -
 Pentoxifylline Pharma Grade, 100 mg/5 mlView Details
Pentoxifylline Pharma Grade, 100 mg/5 mlView Details
6493-05-6
