Ferrous oxalate
- CAS NO.:516-03-0
- Empirical Formula: C2FeO4
- Molecular Weight: 143.86
- MDL number: MFCD00012475
- EINECS: 208-217-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 13:37:16
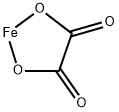
What is Ferrous oxalate?
Description
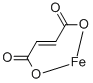
Ferrous oxalate, or iron (II) oxalate, is a chemical compound consisting of one iron (II) ion (Fe2+) and one oxalate ion (C2O42-). It has the chemical formula FeC2O4.
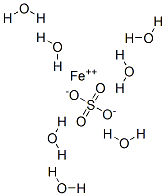
Iron(II) oxalate is more commonly encountered as the dihydrate, FeC2O4·2H2O . Its crystal structure consists of chains of oxalatebridged iron atoms, capped by water molecules.
When heated, it dehydrates and decomposes into carbon dioxide, carbon monoxide, iron oxides and pyrophoric black iron.
Chemical properties
Pale-yellow, crystalline powder; odorless. Soluble in acids; insoluble in water.
The Uses of Ferrous oxalate
Photographic developer, pigment in glass, plastics, paints.
The Uses of Ferrous oxalate
Used as a photographic developer, glass tint, decorative glass colorant, and pigment in plastics, paints, and lacquers.
General Description
Odorless yellow solid. Insoluble in water and denser in water. Sinks in water.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
Inorganic reducing agents, such as Ferrous oxalate, react with oxidizing agents to generate heat and products that may be flammable, combustible, or otherwise reactive.
Hazard
Toxic. Evolves carbon monoxide on heating.
Health Hazard
Inhalation of dust may cause irritation of nose and throat. Ingestion causes burning pain in throat and stomach; mucous membranes turn white; can also cause vomiting, weak pulse, collapse, and death. Dust irritates eyes and may irritate skin on prolonged contact.
Fire Hazard
Special Hazards of Combustion Products: Iron fume or iron oxide fume may form in fire.
Flammability and Explosibility
Not classified
Properties of Ferrous oxalate
| Melting point: | 190°C |
| Density | 2.30 |
| vapor pressure | 0.038Pa at 25℃ |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | Soluble in acids |
| form | Powder |
| Water Solubility | Slightly soluble |
| Sensitive | Hygroscopic |
| Exposure limits | ACGIH: TWA 1 mg/m3 NIOSH: TWA 1 mg/m3 |
| CAS DataBase Reference | 516-03-0(CAS DataBase Reference) |
| EPA Substance Registry System | Iron, [ethanedioato(2-)-.kappa.O1,.kappa.O2]- (516-03-0) |
Safety information for Ferrous oxalate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H312:Acute toxicity,dermal |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P321:Specific treatment (see … on this label). |
Computed Descriptors for Ferrous oxalate
Ferrous oxalate manufacturer
Nithyasri Chemicals (APURVA CHEMICALS)
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde Electrolytic Iron Powder 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 4-HYDROXY BENZYL ALCOHOL 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) S-2-CHLORO PROPIONIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 3-(Hydroxymethyl)benzoate N-Boc-2-chloroethylamine 1-Bromo-2-methoxy-3-nitrobenzene N-Methyl-3-cyclopenten-1-amine 2-Bromo-3-hydroxybenzaldehyde 1H-indazole-5-carboxamideRelated products of tetrahydrofuran


You may like
-
 516-03-0 Iron(II) oxalate 98%View Details
516-03-0 Iron(II) oxalate 98%View Details
516-03-0 -
 Iron(II) oxalate hydrate CAS 516-03-0View Details
Iron(II) oxalate hydrate CAS 516-03-0View Details
516-03-0 -
 Iron(II) oxalate hydrate CAS 516-03-0View Details
Iron(II) oxalate hydrate CAS 516-03-0View Details
516-03-0 -
 Ferrous oxalate 95% CAS 516-03-0View Details
Ferrous oxalate 95% CAS 516-03-0View Details
516-03-0 -
 Ferrous oxalate CAS 516-03-0View Details
Ferrous oxalate CAS 516-03-0View Details
516-03-0 -
 Ferrous Oxalate CASView Details
Ferrous Oxalate CASView Details -
 1260741-78-3 6-Bromo-3-iodo-1-methyl-1H-indazole 98%View Details
1260741-78-3 6-Bromo-3-iodo-1-methyl-1H-indazole 98%View Details
1260741-78-3 -
 2490430-37-8 98%View Details
2490430-37-8 98%View Details
2490430-37-8