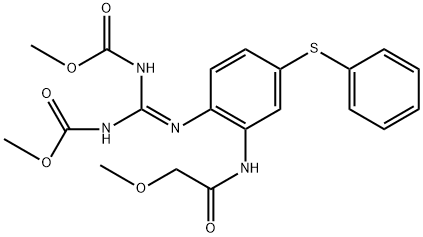
Febantel
Synonym(s):N-{2-[2,3-Bis-(methoxycarbonyl)-guanido]-5-(phenylthio)-phenyl}-2-methoxyacetamide;Febantel
- CAS NO.:58306-30-2
- Empirical Formula: C20H22N4O6S
- Molecular Weight: 446.48
- MDL number: MFCD01738527
- EINECS: 261-205-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-04 16:42:01
What is Febantel?
Description
Febantel [N-2-( [2,3-bis-(methoxycarbonyl)-guanidine] 5- phenylthio)-phenyl) -2-methoxyacetamide] is a new anthelmintic that is highly active against various species of nematodes and cestodes in rodents, sheep, cattle, and horses. In 1978, febantel was introduced to South Africa under the trade name of Rintal? as a sheep drench, and to New Zealand as a sheep and cattle drench, and the USA and Australia as a paste for horse[1].
Chemical properties
Febantel is White to Off-White Solid
Originator
Rintal, Bayer,W. Germany,1979
The Uses of Febantel
Febantel is used as an anthelmintic
The Uses of Febantel
Used as an anthelmintic.
What are the applications of Application
Febantel is an anthelmintic
Definition
ChEBI: Febantel is an aryl sulfide.
Manufacturing Process
2-Amino-5-phenylthiornethoxyacetanilide in methanol solution is heated with N,N'-bis-methoxycarbonyl-isothiourea-S-methyl ether with the addition of a catalytic amount of p-toluenesulfonic acid for three hours with stirring under reflux. The mixture is then filtered hot and after cooling the febantel product crystallizes out. It is filtered off, rinsed with ether and dried under high vacuum to give the final product, melting at 129°C to 130°C.
brand name
Combotel (Bayer AnimalHealth); Negabot Plus Paste (Bayer Animal Health); Oratel (Bayer Animal Health); Rintal (Bayer Animal Health).
Therapeutic Function
Anthelmintic
in vivo
In vivo assays indicated that febantel exhibited potent anthelmintic activity against G. kobayashii with an EC50 value of 0.03 mg/L and 100 % anthelmintic efficacy at 0.1 mg/L after 48 h of exposure. Moreover, in vivo trials also revealed a notable post-treatment effect of febantel, where infected goldfish transferred to drug-free water after short 6-h exposure could still result in full eradication of the worms, indicating febantel might induce persistent perturbations in parasite physiology[1].
in vivo
In vitro assays showed a negative correlation between febantel concentrations and the survival of G. Kobayashi. However, increasing the febantel concentration to 2.0 mg/L did not result in the complete death of all worms. Oral administration of febantel demonstrated limited anthelmintic activity, with only 49 % efficacy at 200 mg/kg body weight daily over five days. Acute toxicity assays revealed that the 48-h LC50 value of febantel was 5.47 mg/L, 182.23 times higher than the 48-h EC50 value, indicating that febantel has a favorable safety profile. However, febantel exposure potentially interfered with hepatic metabolism and oxidative status, as indicated by variations in SOD, GST, and P450 gene expression[1].
References
[1] Thomas, H. “Ovacidal activity of febantel.” New Zealand veterinary journal 27 12 (1979): 273–5.
Properties of Febantel
| Melting point: | 129-130°C |
| Boiling point: | 215°C (rough estimate) |
| Density | 1.2962 (rough estimate) |
| refractive index | 1.6000 (estimate) |
| storage temp. | Sealed in dry,2-8°C |
| solubility | Chloroform (Sparingly) |
| pka | 7.60±0.46(Predicted) |
| form | neat |
| form | Solid |
| color | White to Off-White |
| Merck | 14,3944 |
| InChI | InChI=1S/C20H22N4O6S/c1-28-12-17(25)21-16-11-14(31-13-7-5-4-6-8-13)9-10-15(16)22-18(23-19(26)29-2)24-20(27)30-3/h4-11H,12H2,1-3H3,(H,21,25)(H2,22,23,24,26,27) |
| CAS DataBase Reference | 58306-30-2(CAS DataBase Reference) |
Safety information for Febantel
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
Computed Descriptors for Febantel
| InChIKey | HMCCXLBXIJMERM-UHFFFAOYSA-N |
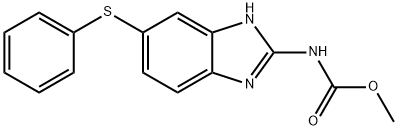
| SMILES | N(C1C=C(SC2C=CC=CC=2)C=CC=1/N=C(\NC(=O)OC)/NC(=O)OC)C(=O)COC |
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 58306-30-2 99%View Details
58306-30-2 99%View Details
58306-30-2 -
 Febantel 58306-30-2 99%View Details
Febantel 58306-30-2 99%View Details
58306-30-2 -
 58306-30-2 Febantel 99%View Details
58306-30-2 Febantel 99%View Details
58306-30-2 -
 Febantel Ep 99%View Details
Febantel Ep 99%View Details -
 Febantel CAS 58306-30-2View Details
Febantel CAS 58306-30-2View Details
58306-30-2 -
 Febantel 98% CAS 58306-30-2View Details
Febantel 98% CAS 58306-30-2View Details
58306-30-2 -
 FEBANTEL CAS 58306-30-2View Details
FEBANTEL CAS 58306-30-2View Details
58306-30-2 -
 Febantel Powder, Bionique PharmaView Details
Febantel Powder, Bionique PharmaView Details
58306-30-2
