Fenbendazole
Synonym(s):Fenbendazole;Fenbendazole Impurity B (PhEur);Methyl (5-chloro-1H-benzimidazol-2-yl)carbamate;Methyl 5-(phenylthio)-2-benzimidazolecarbamate
- CAS NO.:43210-67-9
- Empirical Formula: C15H13N3O2S
- Molecular Weight: 299.35
- MDL number: MFCD00144301
- EINECS: 256-145-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-20 21:15:22
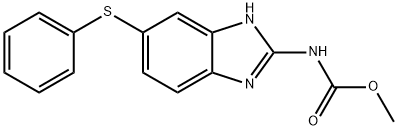
What is Fenbendazole?
Description
Fenbendazole is a benzimidazole anthelmintic. It is active against Giardia in vitro (IC50 = 0.3 μM). Fenbendazole (20 mg/kg) prevents infiltration of parasites into the brain in a rabbit model of E. cuniculi infection. Fenbendazole also activates HIF-1α and prevents oxidative stress-induced death in primary neurons in vitro.
Chemical properties
Off-White Solid
Originator
Panacur,Hoechst,W. Germany,1980
The Uses of Fenbendazole
Fenbendazole is a benzimidazole anthelmintic that is metabolised in mammals to a series of other benzimidazoles including oxfendazole. It is used for the control of gastrointestinal roundworms, lung worms, Nematodes and tape worms. It is the only benzimidazole approved for use in organic livestock production.
The Uses of Fenbendazole
Fenbendazole is a medication used to treat parasites, mainly in dogs. However, studies have found that in a group of mice treated with a combination of fenbendazole and vitamins, tumor growth inhibition was unexpected It may be because it interferes with the uptake of glucose by cancer cells, which is essential for tumor growth and proliferation. It prevents cancer cells from obtaining the glucose needed to grow and divide to inhibit the growth of cancer cells. Studies have found that fenbendazole can kill cancer cells and promote tumor regression in patients with metastatic large B-cell lymphoma, and also plays a role in other metastatic malignant tumors such as bladder cancer and renal cell carcinoma. It is currently being treated in combination with other anti-cancer drugs to study its anti-cancer potential[1-3].
What are the applications of Application
Fenbendazole is a potent CYP1A2 inducer
Definition
ChEBI: Fenbendazole is a member of the class of benzimidazoles that is 1H-benzimidazole which is substituted at positons 2 and 5 by (methoxycarbonyl)amino and phenylsulfanediyl groups, respectively. A broad-spectrum anthelmintic, it is used, particularly in veterinary medicine, for the treatment of nematodal infections. It has a role as an antinematodal drug. It is a member of benzimidazoles, a carbamate ester and an aryl sulfide.
Manufacturing Process
20.9 g of S-methyl-thiourea were dissolved in 27 ml of water with 13.5 ml of chloroformic acid methyl ester. Then, 45.7 ml of 25% sodium hydroxide solution were added dropwise, while stirring, at a temperature of 5°C to 10°C. After having stirred for 20 minutes, the reaction mixture was combined with 27 ml of glacial acetic acid, 100 ml of water and 29 g of 3,4-diaminodiphenyl- thioether. Stirring was continued for 90 minutes at a temperature of 85°C, during which time methyl-mercaptan was separated. After having allowed the whole to cool and stand overnight, the 5-phenylmercaptobenzimidazole- 2-methyl-carbamate that had formed was filtered off with suction. After recrystallization from a mixture of glacial acetic acid and methanol, 14 g of 4-phenylmercapto-benzimidazole-2-methyl-carbamate melting at 233°C were obtained.
brand name
Panacur (Hoechst-Roussel).
Therapeutic Function
Anthelmintic
General Description
Fenbendazole is a thio substituted benzimidazole, which belongs to the group of anthelmintics. It can be widely used in veterinary medicine particularly, in the treatment of helminth infections.
Side Effects
According to the results of toxicological studies, fenbendazole appears to be a safe drug, but up to 5% of people may experience stomach pain or diarrhea if taken in large amounts without interruption. People with severe renal failure or liver failure have a reduced level of drug excretion, and since fenbendazole powder is metabolized mainly in the liver, long-term heavy use can cause an asymptomatic increase in liver enzymes.
Veterinary Drugs and Treatments
Fenbendazole is indicated (labeled) for the removal of the following parasites in dogs: ascarids (Toxocara canis, T. leonina), Hookworms (Ancylostoma caninum, Uncinaria stenocephala), whipworms (Trichuris vulpis), and tapeworms (Taenia pisiformis). It is not effective against Dipylidium caninum. Fenbendazole has also been used clinically to treat Capillaria aerophilia, Filaroides hirthi, and Paragonimus kellicotti infections in dogs.
Fenbendazole is indicated (labeled) for the removal of the following parasites in cattle: Adult forms of: Haemonchus contortus, Ostertagia ostertagi, Trichostrongylus axei, Bunostomum phlebotomum, Nematodirus helvetianus, Cooperia spp., Trichostrongylus colubriformis, Oesophagostomum radiatum, and Dictyocaulus vivaparus. It is also effective against most immature stages of the above listed parasites. Although not approved, it has good activity against Moniezia spp., and arrested 4th stage forms of Ostertagia ostertagi. Fenbendazole is indicated (labeled) for the removal of the following parasites in horses: large strongyles (S. edentatus, S. equinus, S. vulgaris), small strongyles (Cyathostomum spp., Cylicocylus spp., Cylicostephanus spp., Triodontophorus spp.), and pinworms (Oxyuris equi).
Fenbendazole is indicated (labeled) for the removal of the following parasites in swine: large roundworms (Ascaris suum), lungworms (Metastrongylus pair), nodular worms (Oesphagostomum dentatum, O. quadrispinolatum), small stomach worms (Hyostrongylus rubidus), whipworms (Trichuris suis), and kidney worms (Stephanurus dentatus; both mature and immature).
Although not approved, fenbendazole has been used in cats, sheep, goats, pet birds, and llamas.
Mode of action
Fenbendazole is a benzimidazole antiparasitic drug that works at the sub-cellular level preventing cell division. Benzimidazoles bind to the β-tubulin, inhibiting the cell’s microtubule assembly responsible for intracellular transport and required for mitotic cellular division. In effect, it starves the parasite by causing intestinal cell disruption.
References
[1] Gao P, et al. Unexpected antitumorigenic effect of fenbendazole when combined with supplementary vitamins. Journal of the American Association for Laboratory Animal Science : JAALAS, 2008.
[2] Park D, et al. Anti-cancer effects of fenbendazole on 5-fluorouracil-resistant colorectal cancer cells. The Korean Journal of Physiology Pharmacology, 2022; 26: 377-387.[3] Shin Y, et al. Anticancer Evaluation of Methoxy Poly(Ethylene Glycol)-b-Poly(Caprolactone) Polymeric Micelles Encapsulating Fenbendazole and Rapamycin in Ovarian Cancer. International Journal of Nanomedicine, 2023; 2023: 2209—2223.
Properties of Fenbendazole
| Melting point: | 233°C |
| Density | 1.2767 (rough estimate) |
| refractive index | 1.6740 (estimate) |
| storage temp. | 2-8°C |
| Water Solubility | Insoluble in water |
| solubility | DMSO:16.67(Max Conc. mg/mL);55.69(Max Conc. mM) DMF:10.0(Max Conc. mg/mL);33.41(Max Conc. mM) |
| pka | 10.80±0.10(Predicted) |
| form | Solid |
| form | neat |
| color | White to Light yellow |
| Merck | 14,3960 |
| BRN | 759077 |
| InChI | InChI=1S/C15H13N3O2S/c1-20-15(19)18-14-16-12-8-7-11(9-13(12)17-14)21-10-5-3-2-4-6-10/h2-9H,1H3,(H2,16,17,18,19) |
| CAS DataBase Reference | 43210-67-9(CAS DataBase Reference) |
| EPA Substance Registry System | Carbamic acid, N-[6-(phenylthio)-1H-benzimidazol-2-yl]-, methyl ester (43210-67-9) |
Safety information for Fenbendazole
| Signal word | Warning |
| Pictogram(s) |
 Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H373:Specific target organ toxicity, repeated exposure H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Fenbendazole
| InChIKey | HDDSHPAODJUKPD-UHFFFAOYSA-N |
| SMILES | C(OC)(=O)NC1NC2=CC(SC3=CC=CC=C3)=CC=C2N=1 |
Fenbendazole manufacturer
Clickchem Research LLP
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 Fenbendazole 99%View Details
Fenbendazole 99%View Details -
 Fenbendazole 99%View Details
Fenbendazole 99%View Details -
 FENBENDAZOLE 99%View Details
FENBENDAZOLE 99%View Details -
 FENBENDAZOLE 99%View Details
FENBENDAZOLE 99%View Details -
 Fenbendazole 98% CAS 43210-67-9View Details
Fenbendazole 98% CAS 43210-67-9View Details
43210-67-9 -
 Fenbendazole 888 Mg Tablets Wormetel 888View Details
Fenbendazole 888 Mg Tablets Wormetel 888View Details
43210-67-9 -
 FENBENDAZOLE (43210-67-9)View Details
FENBENDAZOLE (43210-67-9)View Details
43210-67-9 -
 Fenbendazole 500mg tabletsView Details
Fenbendazole 500mg tabletsView Details
43210-67-9
