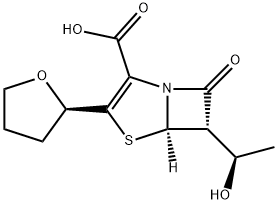
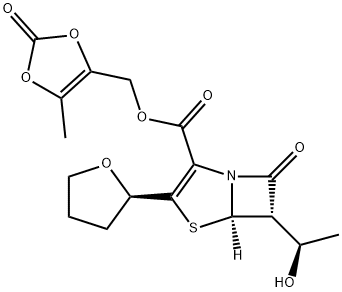
Faropenem sodium hemipentahydrate
- CAS NO.:106560-14-9
- Empirical Formula: C12H15NO5S
- Molecular Weight: 285.32
- MDL number: MFCD00868867
- EINECS: 1312995-182-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-04 17:34:31
What is Faropenem sodium hemipentahydrate?
Description
Farom was launched in Japan for use as an antibiotic against common respiratory tract pathogens. It can be prepared by several related routes of about seven steps starting with tetrahydrofuran-2-thiocarboxylic acid and a silylated azetidinone. It is a broad spectrum oral penem antibiotic that is β-lactamase stable. Farom is the most active β-lactam against anaerobes (more than cefaclor, cefixime and amoxicillin) but also has activity against Gram-positive, Gram-negative and enterobacteriaceae. It is equally active against strains carrying plasmid and chromosome mediated β-lactamases. The short plasma elimination half-life is the result of hydrolysis by renal dehydropeptidase. It is more stable to hydrolysis by extended spectrum β-lactamases than some second and third generation cephalosporins.
Originator
Suntory (Japan)
The Uses of Faropenem sodium hemipentahydrate
Treatmentof bacterial infections.
The Uses of Faropenem sodium hemipentahydrate
Faropenem (cas# 106560-14-9) is a compound useful in organic synthesis.
Definition
ChEBI: 6alpha-[(R)-1-hydroxyethyl]-2-[(R)-tetrahydrofuran-2-yl]pen-2-em-3-carboxylic acid is a faropenem. It is a conjugate acid of a 6alpha-[(R)-1-hydroxyethyl]-2-[(R)-tetrahydrofuran-2-yl]pen-2-em-3-carboxylate.
brand name
Farom
Antimicrobial activity
An orally active penem with a broad spectrum of antibacterial activity, including activity against selected anaerobic pathogens. Although it is active against most enterobacteria, it has reduced activity against Ser. marcescens, Enterobacter spp. and some Providencia spp. It generally retains activity against many Gram-positive organisms, but has no useful activity against Ps. aeruginosa. It has reduced activity against E. faecium, methicillin-resistant Staph. aureus (MRSA) and some strains of coagulase-negative staphylococci. It is stable to hydrolysis by extended spectrum β-lactamases, but is hydrolyzed by carbapenemases. Esterified prodrugs with increased bioavailability have been studied in clinical trials but have not received regulatory approval.
Properties of Faropenem sodium hemipentahydrate
| Boiling point: | 570.2±50.0 °C(Predicted) |
| Density | 1.56±0.1 g/cm3(Predicted) |
| pka | 4.00±0.40(Predicted) |
| CAS DataBase Reference | 106560-14-9 |
Safety information for Faropenem sodium hemipentahydrate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Faropenem sodium hemipentahydrate
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran

You may like
-
 106560-14-9 Faropenem 98%View Details
106560-14-9 Faropenem 98%View Details
106560-14-9 -
 106560-14-9 98%View Details
106560-14-9 98%View Details
106560-14-9 -
 Faropenem sodium hemipentahydrate 95% CAS 106560-14-9View Details
Faropenem sodium hemipentahydrate 95% CAS 106560-14-9View Details
106560-14-9 -
 17604-74-9 3-Pyridineacetonitrile, α-hydroxy- 98+View Details
17604-74-9 3-Pyridineacetonitrile, α-hydroxy- 98+View Details
17604-74-9 -
 Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
67967-07-1 -
 2-Propanamine, 1-chloro-, hydrochloride (9CI) 98+View Details
2-Propanamine, 1-chloro-, hydrochloride (9CI) 98+View Details
5968-21-8 -
 3-Iodophenylacetic acid 1878-69-9 98+View Details
3-Iodophenylacetic acid 1878-69-9 98+View Details
1878-69-9 -
 132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6
