Etofenprox
Synonym(s):2-(4-Ethoxyphenyl)-2-methylpropyl 3-phenoxybenzyl ether
- CAS NO.:80844-07-1
- Empirical Formula: C25H28O3
- Molecular Weight: 376.49
- MDL number: MFCD00210287
- EINECS: 407-980-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-08-21 22:16:43
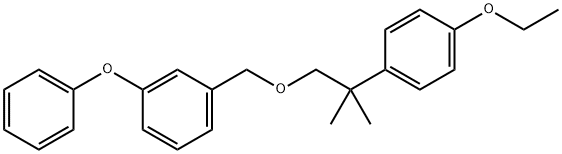
What is Etofenprox ?
Chemical properties
solid
The Uses of Etofenprox
Etofenprox is used to control a wide variety of insects on fruit, tea, soyabeans and many vegetables. It is also used in public health and animal health.
The Uses of Etofenprox
Insecticide.
Definition
ChEBI: An aromatic ether that is the 3-phenoxybenzyl ether of 2-(4-ethoxyphenyl)-2-methylpropan-1-ol.
Agricultural Uses
Insecticide: Approved for use in the U.S. and more than a dozen EU countries.
Trade name
MTI 500®; PUNKASO®; TREBON®; ZOECON® RF-316
Metabolic pathway
Etofenprox is one of the few members of a new class, the non-ester pyrethroids. It lacks the ester bond and therefore is not subject to facile chemical or biochemical hydrolysis. Metabolic options will be limited to oxidative processes. Little has been published in the scientific literature. Its photochemistry has been described and metabolic oxidation products have been noted.
Degradation
Etofenprox is stable in acid and alkaline media for more than 100 days at
80 °C. It is reasonably stable to light under normal conditions of use but it
is photodegradable. Under conditions where allethrin was degraded with
a half-life of 0.5 hours, etofenprox had a half-life of 3 hours (Tsao and Eto,
1990).
The major product was the ester formed by oxidation at the benzylic
carbon atom to form 2-(4-ethoxyphenyl)-2-methylpropyl 3-phenoxybenzoate
(2), clearly the result of hydroxylation and dehydrogenation.
This ester was found to be non-toxic to houseflies. It was cleaved to form 3PBA (5) and the alcohol (6). 6 was degraded via decarboxylation to 7 and
8. A concurrent reaction of etofenprox was O-de-ethylation to the phenol
(3). A similar array of products was seen in both aqueous suspension and
as a thin film on glass. It should be noted that this study did not utilise
radiolabelled compound.
Properties of Etofenprox
| Melting point: | 36.4-38.0° |
| Boiling point: | 445.03°C (rough estimate) |
| Density | 1.1386 (rough estimate) |
| vapor pressure | 32×10-3 Pa (100 °C) |
| refractive index | nD20.2 1.5732 |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | DMSO: 100 mg/mL (265.61 mM) |
| form | neat |
| Water Solubility | <0.001 mg l-1(25 °C) |
| form | Solid |
| color | Off-white to light yellow |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 80844-07-1(CAS DataBase Reference) |
| NIST Chemistry Reference | Etofenprox(80844-07-1) |
| EPA Substance Registry System | Etofenprox (80844-07-1) |
Safety information for Etofenprox
| Signal word | Warning |
| Pictogram(s) |
 Environment GHS09 |
| GHS Hazard Statements |
H362:Reproductive toxicity, effects on or via lactation H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P263:Avoid contact during pregnancy/while nursing. P273:Avoid release to the environment. P391:Collect spillage. Hazardous to the aquatic environment P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Etofenprox
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran

You may like
-
 Ethofenprox 95.00% CAS 80844-07-1View Details
Ethofenprox 95.00% CAS 80844-07-1View Details
80844-07-1 -
 Ethofenprox 98% (GC) CAS 80844-07-1View Details
Ethofenprox 98% (GC) CAS 80844-07-1View Details
80844-07-1 -
 Etofenprox CAS 80844-07-1View Details
Etofenprox CAS 80844-07-1View Details
80844-07-1 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
