Methyl cellulose
Synonym(s):Methocel MC;Methyl 2-hydroxyethyl cellulose;Methyl cellulose;Methylcellulosum
- CAS NO.:9004-67-5
- Empirical Formula: C20H38O11
- Molecular Weight: 454.50912
- MDL number: MFCD00081763
- EINECS: 232-674-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-22 14:18:24
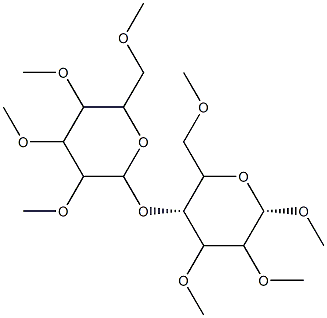
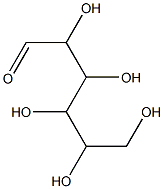
What is Methyl cellulose?
Absorption
Cellulose derivatives considered in this report are virtually unabsorbed and little or no degradation of absorbed and little or no degradation of absorbable products occurs in the human digestive tract. In humans, virtually 100 percent of orally ingested methyl cellulose can be recovered in the feces withihn four days, indicating that absorption does not occur.
Toxicity
Organism: Mouse
Test type: LD50
Route : Intraperitoneal
Reported Dose: 275gm/kg ( 275000mg/kg)
Toxic Effect: Details of toxic effects not reported other than lethal dose value
Organism: Mouse
Test type: LDLo
Route : Intravenous
Reported Dose: 1gm/kg ( 1000mg/kg)
Toxic Effect: Details of toxic effects not reported other than lethal dose value
Chemical properties
Methylcellulose occurs as a white, fibrous powder or granules. It is practically odorless and tasteless. It should be labeled to indicate its viscosity type (viscosity of a 1 in 50 solution).
The Uses of Methyl cellulose
Methyl cellulose is used as a thickener and an emulsifier in various food and cosmetic products; as a gel in advanced cookery and as a lubricant. It is also involved in the treatment of constipation. It acts as a buffer additive in capillary electrophoresis to control electoosmotic flow for improved separations. In paper and textile industries, it is used as a sizing, thereby protecting the fibers from water or oil.
The Uses of Methyl cellulose
Used as a substitute for water-soluble gums; to render paper greaseproof, in adhesives, as thickening agent in cosmetics, as protective colloid in emulsions, as binder and stabilizer in foods. As fat replacer in the formulation of dietetic foods. Pharmaceutic aid (suspending agent).
The Uses of Methyl cellulose
Methylcellulose is a gum composed of cellulose in which the meth- oxyl groups replace the hydroxyl groups. it is soluble in cold water but insoluble in hot water. solutions increase in viscosity upon heating, gel at 50–55°c, and liquefy upon cooling. it is used in baked goods for moisture retention, and in fruit pie fillings for the reduction of water absorption into the pie crust during baking. it is also used in breaded shrimp where it functions to form an oil barrier film.
Indications
Solutions containing methyl cellulose are used as substitute for tears or saliva if the natural production of these fluids is disturbed. It is also used or constipation, diverticulosis, hemorrhoids and irritable bowel syndrome. Used in the manufacture of capsules in nutritional supplements. Its edible and nontoxic properties provide a vegetarian alternative to the use of gelatin.
Background
Methyl cellulose polymer consisting of numerous linked glucose molecules used as a stabiliser, thickener and emulsifier for foodstuffs and cosmetics. The Degree of Substitution (DS) of a given form of methyl cellulose is defined as the average number of substituted hydroxyl groups per glucose with a theoretical maximum of 3, however more typical values are 1.3 2.6. Methyl cellulose is a hydrophilic white powder in pure form and dissolves in cold (but not in hot) water, forming a clear viscous solution or gel. It is available under a variety of trade names as a treatment for constipation. Like cellulose, it is not digestible, not toxic, and not allergenic
Production Methods
Methylcellulose is prepared from wood pulp (cellulose) by treatment with alkali followed by methylation of the alkali cellulose with methyl chloride. The product is then purified and ground to powder form.
Preparation
Methyl cellulose was prepared from wood pulp or cotton by treatment with alkali and methylation of alkali cellulose with methyl chloride.
brand name
Cologel (Lilly); Methocel A (Dow Chemical).
General Description
Odorless white or creamy white fibrous powder. Tasteless.
Air & Water Reactions
Methyl cellulose is hygroscopic. Swells in water to a viscous, colloidal solid. Slightly water soluble.
Reactivity Profile
Methyl cellulose is incompatible with strong oxidizing agents. Methyl cellulose is also incompatible with aminacrine HCl, chlorocresol, mercuric chloride, phenol resorcinol, tannic acid and silver nitrate.
Fire Hazard
Flash point data for Methyl cellulose are not available; however, Methyl cellulose is probably combustible.
Pharmaceutical Applications
Methylcellulose is widely used in oral and topical pharmaceutical
formulations;
In tablet formulations, low- or medium-viscosity grades of
methylcellulose are used as binding agents, the methylcellulose
being added either as a dry powder or in solution.Highviscosity
grades of methylcellulose may also be incorporated in
tablet formulations as a disintegrant.Methylcellulose may be added to a tablet formulation to produce sustained-release
preparations.
Tablet cores may also be spray-coated with either aqueous or
organic solutions of highly substituted low-viscosity grades of
methylcellulose to mask an unpleasant taste or to modify the release
of a drug by controlling the physical nature of the granules.
Methylcellulose coats are also used for sealing tablet cores prior to
sugar coating.
Low-viscosity grades of methylcellulose are used to emulsify
olive, peanut, and mineral oils.They are also used as suspending
or thickening agents for orally administered liquids, methylcellulose
commonly being used in place of sugar-based syrups or other
suspension bases.Methylcellulose delays the settling of suspensions
and increases the contact time of drugs, such as antacids, in the
stomach.
High-viscosity grades of methylcellulose are used to thicken
topically applied products such as creams and gels.
In ophthalmic preparations, a 0.5–1.0% w/v solution of a highly
substituted, high-viscosity grade of methylcellulose has been used as
a vehicle for eye drops.However, hypromellose-based formulations
are now preferred for ophthalmic preparations. Methylcellulose
is also used in injectable formulations.
Therapeutically, methylcellulose is used as a bulk laxative; it has
also been used to aid appetite control in the management of obesity,
but there is little evidence supporting its efficacy.
Biochem/physiol Actions
Methyl cellulose is known to have a molecular size that might pass through placenta. It is useful in visualizing small bowel loops through its overlaps.
Pharmacokinetics
It increases the bulk in your stool, an effect that helps to cause movement of the intestines. It also works by increasing the amount of water in the stool, making the stool softer and easier to pass.
Safety Profile
A poison by intraperitoneal route. When heated to decomposition it emits acrid smoke and irritating fumes.
Safety
Methylcellulose is widely used in a variety of oral and topical
pharmaceutical formulations. It is also extensively used in cosmetics
and food products, and is generally regarded as a nontoxic,
nonallergenic, and nonirritant material.
Following oral consumption, methylcellulose is not digested or
absorbed and is therefore a noncaloric material. Ingestion of
excessive amounts of methylcellulose may temporarily increase
flatulence and gastrointestinal distension.
In the normal individual, oral consumption of large amounts of
methylcellulose has a laxative action and medium- or high-viscosity
grades are therefore used as bulk laxatives.
Esophageal obstruction may occur if methylcellulose is swallowed
with an insufficient quantity of liquid. Consumption of large
quantities of methylcellulose may additionally interfere with the
normal absorption of some minerals. However, this and the other
adverse effects discussed above relate mainly to the use of
methylcellulose as a bulk laxative and are not significant factors
when methylcellulose is used as an excipient in oral preparations.
Methylcellulose is not commonly used in parenteral products,
although it has been used in intra-articular and intramuscular
injections. Studies in rats have suggested that parenterally
administered methylcellulose may cause glomerulonephritis and
hypertension.Methylcellulose is considered to be toxic by the
intraperitoneal route of administration.
The WHO has not specified an acceptable daily intake of
methylcellulose since the level of use in foods was not considered to
be a hazard to health.
LD50 (mouse, IP): 275 g/kg
Metabolism
Reported that when methylcellulose was given iv to dog and rabbit , aside from effect upon circulating blood, inability of body to degrade substance led to its retention & accumulation in liver, spleen, lymph nodes, kidney, and vascular walls.
Storage
Methylcellulose powder is stable, although slightly hygroscopic.
The bulk material should be stored in an airtight container in a cool,
dry place.
Solutions of methylcellulose are stable to alkalis and dilute acids
at pH 3–11, at room temperature. At pH less than 3, acid-catalyzed
hydrolysis of the glucose–glucose linkages occurs and the viscosity
of methylcellulose solutions is reduced.On heating, solution
viscosity is reduced until gel formation occurs at approximately
50°C;
Methylcellulose solutions are liable to microbial spoilage and
antimicrobial preservatives should therefore be used. Solutions may
also be sterilized by autoclaving, although this process can decrease
the viscosity of a solution.The change in viscosity after
autoclaving is related to solution pH. Solutions at pH less than 4 had viscosities reduced by more than 20% subsequent to autoclaving.
Incompatibilities
chlorocresol; mercuric chloride; phenol; resorcinol; tannic acid;
silver nitrate; cetylpyridinium chloride; p-hydroxybenzoic acid; paminobenzoic
acid; methylparaben; propylparaben; and butylparaben.
Salts of mineral acids (particularly polybasic acids), phenols, and
tannins will coagulate solutions of methylcellulose, although this
can be prevented by the addition of ethanol (95%) or glycol
diacetate. Complexation of methylcellulose occurs with highly
surface-active compounds such as tetracaine and dibutoline sulfate.
High concentrations of electrolytes increase the viscosity of
methylcellulose mucilages owing to the ‘salting out’ of methylcellulose.
With very high concentrations of electrolytes, the methylcellulose
may be completely precipitated in the form of a discrete or
continuous gel. Methylcellulose is incompatible with strong
oxidizing agents.
Regulatory Status
GRAS listed. Accepted as a food additive in the USA, Europe and Japan. Included in the FDA Inactive Ingredients Database (sublingual tablets; IM injections; intrasynovial injections; nasal preparations; ophthalmic preparations; oral capsules, oral suspensions, and oral tablets; topical and vaginal preparations). Included in nonparenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients.Reported in the EPA TSCA inventory.
Properties of Methyl cellulose
| Melting point: | 290-305 °C |
| Density | 1.01 g/cm3(Temp: 70 °C) |
| FEMA | 2696 | METHYL CELLULOSE |
| storage temp. | Room Temperature |
| solubility | Practically insoluble in hot water, in acetone, in anhydrous ethanol and in toluene. It dissolves in cold water giving a colloidal solution. |
| form | powder |
| color | White, yellow-white or grayish-white |
| Odor | odorless |
| Water Solubility | SOLUBLE IN COLD WATER |
| Merck | 14,6040 |
| Dielectric constant | 3.0(Ambient) |
| Stability: | Stable. Incompatible with strong oxidizing agents, bleach, perchloric acid, nitric acid, perchlorates, alkali nitrates, alkali nitrites, calcium oxide. |
| EPA Substance Registry System | Methylcellulose (9004-67-5) |
Safety information for Methyl cellulose
Computed Descriptors for Methyl cellulose
Methyl cellulose manufacturer
Pharma Links
Bazayan & Co.
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran






You may like
-
 Methyl cellulose, Viscosity: 4000cPs CAS 9004-67-5View Details
Methyl cellulose, Viscosity: 4000cPs CAS 9004-67-5View Details
9004-67-5 -
 Methyl cellulose, Viscosity: 4000cPs CAS 9004-67-5View Details
Methyl cellulose, Viscosity: 4000cPs CAS 9004-67-5View Details
9004-67-5 -
 Methyl cellulose, Viscosity: 4000cPs CAS 9004-67-5View Details
Methyl cellulose, Viscosity: 4000cPs CAS 9004-67-5View Details
9004-67-5 -
 Methyl cellulose, Viscosity: 1600cPs CAS 9004-67-5View Details
Methyl cellulose, Viscosity: 1600cPs CAS 9004-67-5View Details
9004-67-5 -
 Methyl cellulose, Viscosity: 1600cPs CAS 9004-67-5View Details
Methyl cellulose, Viscosity: 1600cPs CAS 9004-67-5View Details
9004-67-5 -
 Methyl cellulose, Viscosity: 400cPs CAS 9004-67-5View Details
Methyl cellulose, Viscosity: 400cPs CAS 9004-67-5View Details
9004-67-5 -
 Methyl cellulose, Viscosity: 400cPs CAS 9004-67-5View Details
Methyl cellulose, Viscosity: 400cPs CAS 9004-67-5View Details
9004-67-5 -
 Methyl cellulose, Viscosity: 400cPs CAS 9004-67-5View Details
Methyl cellulose, Viscosity: 400cPs CAS 9004-67-5View Details
9004-67-5
