Ethylene thiourea
Synonym(s):N,N′-Ethylenethiourea;2-Imidazolidinethione;2-Mercaptoimidazoline;2-Thioxoimidazolidine;N,N′-Ethylenethiourea
- CAS NO.:96-45-7
- Empirical Formula: C3H6N2S
- Molecular Weight: 102.16
- MDL number: MFCD00005276
- EINECS: 202-506-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-15 16:23:17
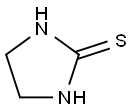
What is Ethylene thiourea?
Description
Ethylenethiourea, a thiourea derivative, is a rubber chemical. It has caused contact dermatitis mainly in rubber workers.
Chemical properties
Ethylene thiourea is a white to light green, needle-like crystalline solid with a faint amine odor. It is very soluble in hot water;slightly soluble in cold water, methanol, ethanol, ethylene glycol, pyridine, acetic acid, and naphtha; and insoluble in acetone, ether, chloroform, and benzene.When heated to decomposition, ethylene thiourea emits toxic fumes of nitrogen oxides (NOx) and sulfur oxides (SOx).

Ethylene thiourea is available in the United States as crystals, as a powder, as an 80% dispersion of the powder in oil, or encapsulated in a matrix of compatible elastomers.
The Uses of Ethylene thiourea
elastomer accelerator;chlorinated polyethylene (CPE) rubber vulcanizing accelerator agent.
Ethylene thiourea is used primarily as an accelerator for vulcanizing polychloroprene (Neoprene®) and polyacrylate rubbers. Neoprene rubbers are used almost exclusively in industrial applications,e.g.. for mechanical and automotive products, in wire and cable production, in construction,and in adhesives. Polyacrylate rubbers are used in products such as seals, o-rings, and gaskets for automotive and aircraft applications. Ethylene thiourea is used in the manufacture of ethylene-bisdithiocarbamate pesticides, such as Maneb®, Mancozeb®, Metiram®,and Zineb®.Ethylene thiourea is also used in electroplating baths, as an intermediate in antioxidant production, in dyes, pharmaceuticals,and synthetic resins. However, there is no evidence that the compound is used commercially for any of these purposes (IARC V.7, 1974; Sax, 1987).
The Uses of Ethylene thiourea
2-Imidazolidinethione is a pesticide used in production of fruits and vegetables. It is also used as a new contrast agent for MRI studies based on proton chemical exchange dependent saturation transfe r. 2-Imidazolidinethione is also a corrosion inhibitor due to the adsorption of the mol. species.
The Uses of Ethylene thiourea
Accelerator in the curing of polychloroprene (neoprene) and polyacrylate rubber; intermediate in the manufacture of antioxidants, insecticides, fungicides, dyes, pharmaceuticals, and synthetic resins
Definition
ChEBI: Ethylenethiourea is a member of imidazolidines.
General Description
White to pale green crystals or an off-white solid. Odorless when pure, but technical product may have an amine odor.
Air & Water Reactions
Slightly soluble in water.
Reactivity Profile
Ethlenethiourea may be sensitive to prolonged exposure to light. Incompatible with acids, diazo and azo compounds, halocarbons, isocyanates, aldehydes, alkali metals, nitrides, hydrides, and other strong reducing agents. Reactions with these materials generate heat and in many cases hydrogen gas. May react with acids to liberate hydrogen sulfide.
Hazard
Questionable carcinogen.
Health Hazard
Ethylene thiourea (ETU) is an antithyroid substance and animal carcinogen.
Fire Hazard
Ethylene thiourea is combustible.
Flammability and Explosibility
Non flammable
Contact allergens
Ethylene thiourea, a thiourea derivative, is a rubber chemical. It caused contact dermatitis mainly in rubber workers.
Potential Exposure
Ethylene thiourea is used extensively as an accelerator in the curing of polychloroprene (Neoprene) and other elastomers; as a vulcanizing accelerator in rubber processing; in electroplating baths. In addition, exposure to ethylene thiourea also results from the very widely used ethylene bisdithiocarbamate fungicides. Ethylene thiourea may be present as a contaminant in the ethylene bisdithiocarbamate fungicides and can also be formed when food containing the fungicides is cooked
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.Medical observation is recommended for 24- 48 h after breathing overexposure, as pulmonary edema may bedelayed. As first aid for pulmonary edema, a doctor orauthorized paramedic may consider administering a corticosteroid spray.
Carcinogenicity
Ethylene thiourea is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in experimental animals.
Storage
Color Code—Blue: Health Hazard/Poison: Storein a secure poison location. Prior to working with thischemical you should be trained on its proper handling andstorage. Store in tightly closed containers in a cool, well-ventilated area away from strong oxidizers, acids, acidanhydrides, acrolein. Store in a refrigerator or a cool, dryplace. A regulated, marked area should be establishedwhere this chemical is handled, used, or stored in compliance with OSHA Standard 1910.1045.
Shipping
UN2811 Toxic solids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required
Purification Methods
Crystallise it from EtOH or amyl alcohol. [Beilstein 24 III/IV 22.]
Incompatibilities
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, acid anhydrides, and acrolein
Waste Disposal
Incineration in a furnace equipped with afterburner and scrubber.
Properties of Ethylene thiourea
| Melting point: | 196-200 °C(lit.) |
| Density | 1.41~1.45 |
| vapor pressure | <1 hPa (25 °C) |
| refractive index | 1.5500 (estimate) |
| Flash point: | 252 °C |
| storage temp. | Store below +30°C. |
| solubility | 8g/l |
| form | Powder |
| pka | 15.01±0.20(Predicted) |
| color | White |
| Odor | wh. to pale green crystals, faint amine odor |
| Water Solubility | 19 g/L (20 ºC) |
| Merck | 14,3803 |
| BRN | 106275 |
| Boiling point: | 240°C (1010 hPa) |
| CAS DataBase Reference | 96-45-7(CAS DataBase Reference) |
| NIST Chemistry Reference | 2-Imidazolidinethione(96-45-7) |
| IARC | 3 (Vol. Sup 7, 79) 2001 |
| EPA Substance Registry System | Ethylene thiourea (96-45-7) |
Safety information for Ethylene thiourea
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H351:Carcinogenicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Ethylene thiourea
| InChIKey | PDQAZBWRQCGBEV-UHFFFAOYSA-N |
Ethylene thiourea manufacturer
Sagar Chemicals, Mumbai
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 Ethylene thiourea CAS 96-45-7View Details
Ethylene thiourea CAS 96-45-7View Details
96-45-7 -
 Ethylenethiourea CAS 96-45-7View Details
Ethylenethiourea CAS 96-45-7View Details
96-45-7 -
 2-Imidazolidinethione CAS 96-45-7View Details
2-Imidazolidinethione CAS 96-45-7View Details
96-45-7 -
 Powder Ethylene Thiourea, Packaging Type: Bags/Drums, Packaging Size: 25/50 KgView Details
Powder Ethylene Thiourea, Packaging Type: Bags/Drums, Packaging Size: 25/50 KgView Details
96-45-7 -
 QUREACC ETU (Ethylene Thiourea)View Details
QUREACC ETU (Ethylene Thiourea)View Details
96-45-7 -
 MG ETU 75 ETHYLENE THIOUREAView Details
MG ETU 75 ETHYLENE THIOUREAView Details
96-45-7 -
 ETHYLENE THIOUREA (96-45-7)View Details
ETHYLENE THIOUREA (96-45-7)View Details
96-45-7 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0
