1,1'-Carbonyldiimidazole
Synonym(s):1,1′-Carbonyldiimidazole;1,1′-Carbonyl-diimidazole;CDI
- CAS NO.:530-62-1
- Empirical Formula: C7H6N4O
- Molecular Weight: 162.15
- MDL number: MFCD00005286
- EINECS: 208-488-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-10-21 20:59:21
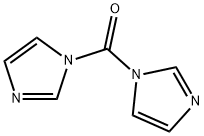
What is 1,1'-Carbonyldiimidazole?
Description
1,1'-Carbonyldiimidazole (CDI) is a highly efficient coupling reagent commonly used in peptide synthesis and organic chemistry. CDI can be used to activate carboxylic acids. These activated carboxylic acids react with nucleophiles to form products such as amides, esters, ureas, and carbamates. CDI reactions do not usually require a base because the released imidazole anion acts as the base. CDI is humidity sensitive and readily reacts with H2O to form CO2.
Chemical properties
The substance appears as a white to slightly pale-yellow crystalline powder with a melting point range of 115.5-116°C. It exhibits poor solubility in water, while displaying solubility in alcohol and ether.
The Uses of 1,1'-Carbonyldiimidazole
Peptide coupling reagent1,1'-Carbonyldiimidazole acts as a coupling reagent and utilized for coupling of amino acids in order to prepare peptide in organic synthesis. It is also used in the preparation of beta-keto sulfones, sulfoxides and beta-enamino acid derivatives. It is used to convert alcohols and amines into carbamates, esters, and ureas. It is involved in the preparation of formylized imidazole by reaction with formic acid. Further, it is used in the synthesis of dipolar polyamides compounds. In addition to this, it is considered as an equivalent of phosgene and used to prepare asymmetric bis alkyl carbonate.
The Uses of 1,1'-Carbonyldiimidazole

The acid (152 mg, 0.801 mmol) was added to a solution of CDI (130 mg, 0.801 mmol) in dry THF (1 mL) at RT under N2. The mixture was stirred for 90 min at RT, after which time it was added over 25 min to a solution of the amine (200 mg, 0.400 mmol) in THF (1.5 mL) at RT under N2. The reaction mixture was stirred at RT for 16 h. Additional acid (152 mg, 0.801 mmol) and CDI (130 mg, 0.801 mmol) in dry THF (1 mL) was added slowly at RT. The reaction mixture was stirred at RT for 2 h. TFA (1 mL, 26.1 mmol) was added at RT, and the mixture was stirred 22 h. Additional TFA (1 mL, 26.1 mmol) was added, and the mixture was stirred 18 h. The reaction mixture was then stirred at 80 C for 160 min. The mixture was diluted with H2O and extracted with EtOAc. The org phase was washed with brine, dried (MgSO4), and concentrated to give an oil. The oil was purified by Prep LC (12 g silica, 100% DCM to 90:9:1 DCM/MeOH/NH3) to provide the product as a white solid. [158 mg, 80%]
The Uses of 1,1'-Carbonyldiimidazole
CDI, also known as 1,1'-carbonyldiimidazole, is a commonly used peptide coupling reagent in the synthesis of peptides and nucleoside triphosphates. It usually contains approximately 10% imidazole and reacts rapidly with carboxylic acids to produce acyl imidazoles. Later, it readily reacts with amines to create amides. In addition, CDI is useful in converting alcohols and amines into a range of chemical compounds, including carbamates, esters, and ureas.
What are the applications of Application
N,N′-Carbonyldiimidazole is a versatile coupling agent used in the synthesis of dipolar polyamides
Preparation
1,1'-Carbonyldiimidazole has been prepared by the reaction of imidazole and phosgene in anhydrous benzene and anhydrous tetrahydrofuran. It has also been obtained by the reaction of 1-(trimethylsilyl)imidazole and phosgene in anhydrous benzene, but that method offers no advantages that justify the more extensive preparative effort required.
DOI: 10.15227/orgsyn.048.0044
General Description
1,1′-Carbonyldiimidazole (N,N′-carbonyldiimidazole) is a versatile peptide-forming reagent.
Purification Methods
Crystallise it from *benzene or tetrahydrofuran in a dry-box and store it dry. [Hearn Methods Enzymol 135 102 1987, Beilstein 23/4 V 245.]
Precautions
1,1'-Carbonyldiimidazole is incompatible with oxidizing agents and strong acids and should not be stored or handled near them. Store in a refrigerator, under inert gas and protected from moisture. This substance is heat sensitive and moisture sensitive.
Properties of 1,1'-Carbonyldiimidazole
| Melting point: | 117-122 °C(lit.) |
| Boiling point: | 288.83°C (rough estimate) |
| Density | 1.303 g/mL at 25 °C |
| vapor pressure | 0.006-0.012Pa at 20-25℃ |
| refractive index | n20/D1.434 |
| storage temp. | 2-8°C |
| solubility | Soluble in dimethylformamide. |
| form | Crystalline Powder |
| pka | 2.90±0.10(Predicted) |
| appearance | White cystalline solid |
| color | White to light cream |
| PH Range | 7.7 at 50 g/l |
| PH | 7.7 (50g/l, H2O, 20℃) |
| Water Solubility | Insoluble in water |
| Sensitive | Moisture Sensitive |
| Merck | 14,1819 |
| BRN | 6826 |
| Stability: | Stable, but moisture-sensitive. Incompatible with acids, strong oxidizing agents, water. |
| CAS DataBase Reference | 530-62-1(CAS DataBase Reference) |
| EPA Substance Registry System | 1H-Imidazole, 1,1'-carbonylbis- (530-62-1) |
Safety information for 1,1'-Carbonyldiimidazole
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 1,1'-Carbonyldiimidazole
| InChIKey | PFKFTWBEEFSNDU-UHFFFAOYSA-N |
1,1'-Carbonyldiimidazole manufacturer
KP INNOVATIVE
VJ CHEMTRADE PVT LTD
HESTUR PHARMA PRIVATE LIMITED
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 Carbonyldiimidazole 98%View Details
Carbonyldiimidazole 98%View Details -
 Carbonyldiimidazole 98%View Details
Carbonyldiimidazole 98%View Details -
 Carbonyldiimidazole 98%View Details
Carbonyldiimidazole 98%View Details -
 Carbonyldiimidazole 98%View Details
Carbonyldiimidazole 98%View Details -
 530-62- 1 99%View Details
530-62- 1 99%View Details
530-62- 1 -
 N,N-Carbonyl Diimidazole CASView Details
N,N-Carbonyl Diimidazole CASView Details -
 N N Carbonyldiimidazole Cdi and DMDO CLView Details
N N Carbonyldiimidazole Cdi and DMDO CLView Details
530-62-1 -
 N N-CarbonyldiimidazoleView Details
N N-CarbonyldiimidazoleView Details
530-62-1
