Eptifibatide
- CAS NO.:188627-80-7
- Empirical Formula: C35H49N11O9S2
- Molecular Weight: 831.97
- MDL number: MFCD05662245
- EINECS: 641-366-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-13 11:39:26
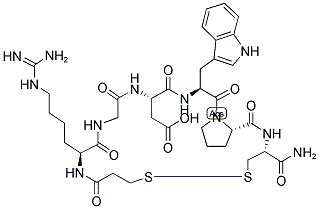
What is Eptifibatide?
Description
Eptifibatide is a reversible antagonist of the glycoprotein llb/llla complex, a specific platelet adhesion receptor that plays a central role in the cascade of thrombus formation by allowing mediators such as fibrinogen or von Willbrand factor to cross-link adjacent platelets and to give rise to aggregation. In diverse animal experimental models of arterial thrombosis, treatment with Eptifibatide resulted in an enhanced lysis of occlusive thrombus and a restoration of arterial blood flow. In clinical trials involving patients with acute coronary syndromes, Eptifibatide demonstrated a significant decrease in the incidence of death or nonfatal myocardial infarction at 30 days. In other trials in patients undergoing percutaneous coronary intervention (PCI), it showed a positive trend. Eptifibatide has the advantage of being short acting, its antiplatelet effect being rapidly reversible.
Originator
Cor Therapeutics (US)
The Uses of Eptifibatide
Eptifibatide is a cyclical heptapeptide with anticoagulant activity. Eptifibatide selectively and reversibly binds to and blocks the platelet glycoprotein IIb/IIIa receptor. This prevents the binding of fibrinogen, von Willebrand factor, and other adhesive ligands and leads to an inhibition of platelet aggregation and prevents thrombus development. It is an efficient peptide drug to reduce the risk of cardiac ischemic events, however has a short half-life. Therefore, antithrombotic agents like eptifibatide are required to become improved with a protected and targeted delivery system such as using nano-liposomes to the site of thrombus.
Definition
ChEBI: Eptifibatide is a synthetic homodetic cyclic peptide comprising N(alpha)-(3-sulfanylpropanoyl)homoarginyl, glycyl, aspartyl, tryptophyl, prolyl and cysteinamide residues connected in sequence and cyclised via a disulfide bond. Derived from a protein found in the venom of the southeastern pygmy rattlesnake, Sistrurus miliarus barbouri, eptifibatide is an anti-coagulant that inhibits platelet aggregation by selectively blocking the platelet glycoprotein IIb/IIIa receptor, so preventing the binding of fibrinogen, von Willebrand factor, and other adhesive ligands. It is used in the management of unstable angina and in patients undergoing coronary angioplasty and stenting procedures. It has a role as a platelet aggregation inhibitor and an anticoagulant. It is an organic disulfide, a macrocycle and a homodetic cyclic peptide.
brand name
Integrilin
General Description
Eptifibatide (188627-80-7) is a syntheticcyclic heptapeptide that acts as a GPIIb/IIIa receptor antagonist,thus causing inhibition of platelet aggregation. Itsstructure is based on the natural product barbourin, a peptideisolated from the venom of a pygmy rattlesnake (Sistrurusmilarud barbouri). As part of the structure, there is a sequenceRGD that can bind to the RGD receptor found onplatelets and block its ability to bind with fibrinogen. Thisagent is used in the treatment of unstable angina and for angioplasticcoronary interventions.
Biological Activity
Eptifibatide is a potent glycoprotein IIb/IIIa antagonist (GPIIb/IIIa; Kd = 120 nM) that inhibits platelet aggregation. Eptifibatide prevents binding of the adhesion proteins fibrinogen and von Willebrand factor to GPIIb/IIIa on the surface of activated platelets to prevent aggregation and thrombus formation. It inhibits ADP-induced citrated blood aggregation (IC50 = 0.11-0.22 μg/ml) in vitro and in vivo (IC50 = 52 μg/ml in porcine plasma). Formulations containing eptifibatide have been used to reduce risk of thrombolysis in myocardial infarction in patients undergoing percutaneous coronary intervention.
Mechanism of action
Eptifibatide reversibly inhibits platelet aggregation by preventing the binding of fibrinogen, von Willebrand factor and other adhesive ligands to the glycoprotein (GP) IIb/IIIa receptors. When administered intravenously, eptifibatide inhibits ex vivo platelet aggregation in a dose- and concentration-dependent manner.
www.accessdata.fda.gov
Clinical Use
Eptifibatide is a cyclic heptapeptide composed of six amino acids and one mercaptopropionyl residue. The cyclization is completed via a disulfide linkage between the cysteine and the mercaptopropionyl moieties. The lysine-glycine-aspartate component of eptifibatide is highly specific for the GPIIb/IIIa receptor, with low binding affinity, as indicated by the rapid dissociation constant. Because of this, eptifibatide is a reversible, parenterally administered antagonist of platelet aggregation.
Drug interactions
Potentially hazardous interactions with other drugs Iloprost: increased risk of bleeding.
Metabolism
Renal excretion accounts for approximately 50% of total body clearance of eptifibatide; approximately 50% of the amount cleared is excreted unchanged in the urine.
storage
Store at -20°C
Properties of Eptifibatide
| Density | 1.60±0.1 g/cm3(Predicted) |
| storage temp. | Sealed in dry,Store in freezer, under -20°C |
| solubility | DMF: 30 mg/ml,DMSO: 14 mg/ml,Ethanol: 5 mg/ml,PBS (pH 7.2): 5 mg/ml |
| pka | 4.01±0.10(Predicted) |
| form | A crystalline solid |
| color | White to off-white |
| Water Solubility | Soluble to 5 mg/ml in water |
| CAS DataBase Reference | 188627-80-7 |
Safety information for Eptifibatide
| Signal word | Warning |
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H373:Specific target organ toxicity, repeated exposure |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P314:Get medical advice/attention if you feel unwell. |
Computed Descriptors for Eptifibatide
| InChIKey | CZKPOZZJODAYPZ-ITVGJGJRNA-N |
| SMILES | N12CCC[C@H]1C(=O)N[C@@H](CSSCCC(=O)N[C@@H](CCCCNC(N)=N)C(=O)NCC(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC1C3C=CC=CC=3NC=1)C2=O)C(N)=O |&1:4,8,17,33,41,r| |
Eptifibatide manufacturer
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran



You may like
-
 188627-80-7 Eptifibatide 98%View Details
188627-80-7 Eptifibatide 98%View Details
188627-80-7 -
 188627-80-7 98%View Details
188627-80-7 98%View Details
188627-80-7 -
 Eptifibatide 98%View Details
Eptifibatide 98%View Details
188627-80-7 -
 Eptifibatide 95% CAS 188627-80-7View Details
Eptifibatide 95% CAS 188627-80-7View Details
188627-80-7 -
 Eptifibatide 98% (LCMS/HPLC) CAS 188627-80-7View Details
Eptifibatide 98% (LCMS/HPLC) CAS 188627-80-7View Details
188627-80-7 -
 Eptifibatide CASView Details
Eptifibatide CASView Details -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1