SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H413:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. |
COMPUTED DESCRIPTORS
| Molecular Weight | 429.9 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 11 |
| Exact Mass | 429.1455339 g/mol |
| Monoisotopic Mass | 429.1455339 g/mol |
| Topological Polar Surface Area | 74.7 Ų |
| Heavy Atom Count | 30 |
| Formal Charge | 0 |
| Complexity | 525 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 2 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
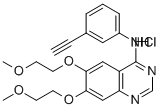
Erlotinib hydrochloride (Tarceva), a member of a class of targeted anticancer drugs that inhibit the activity of the epidermal growth factor receptor, was approved by the US FDA in November 2004 for the treatment of advanced non-small-cell lung cancer after failure of at least one prior chemotherapy regimen. It is the first such drug to demonstrate an increase in survival in Phase III trials in patients with advanced non-small-cell lung cancer. Erlotinib is an EGFR tyrosine kinase inhibitor used to treat certain small cell lung cancers or advanced metastatic pancreatic cancers.
RELATED SUPPLIERS
ALS India Life Sciences Pvt. Ltd
2Y
product:N-(3-Ethynylphenyl)-6,7-bis(2-methoxyethoxy)quinazolin-4-amine hydrochloride(Erlotinib HCl) 99.34%