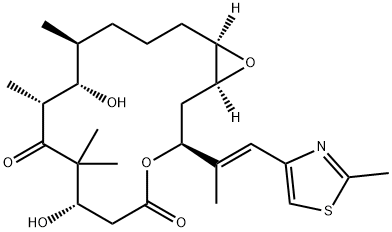
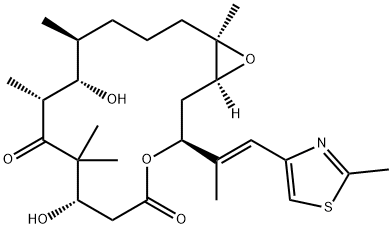
Epothilone A
Synonym(s):EpoA
- CAS NO.:152044-53-6
- Empirical Formula: C26H39NO6S
- Molecular Weight: 493.66
- MDL number: MFCD26960884
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
What is Epothilone A?
Description
Epothilone-A is the first of a new class of compounds that can fight cancer cells that resist conventional chemotherapy. Altough the epothilones work in a manner similar to paclitaxel, they have simpler structures, are easier to make, and dissolve better in water.
The Uses of Epothilone A
The epothilones are a novel class of antineoplastic agents possessing antitubulin activity. Epothilone A, B isolated from Sorangium cellulosum was associated with cell cycle arrest at G2/M transition and apoptosis that was resisted with overexpression of β-tubulin and P-glycoprotein in human bladder carcinoma cell.
The Uses of Epothilone A
Epothilone A is a microtubule inhibitor isolated from the myxobacteria, Sorangium cellulosum. Epothilone A acts by stabilising microtubule formation at the taxol binding site, and causes cell cycle arrest at the G2/M transition, leading to cytotoxicity. Epothilone A has been investigated in clinical trials as an antitumour agent.
What are the applications of Application
Epothilone A is an antitubulin agent
Definition
ChEBI: An epithilone that is epothilone C in which the double bond in the macrocyclic lactone ring has been oxidised to the corresponding epoxide (the 13R,14S diastereoisomer).
Biological Activity
epothilone a (epo a) is a naturally occurring microtubule-stabilizing macrolide that was first isolated from the myxobacterium sorangium cellulosum. the ic50 value of epothilone a in hct-116 cell line is 4.4 nm [1].it has been found that the skov-3 human ovarian cancer cells were susceptible to epothilone a with ic50 value of 20.4 ± 1.4 nm [2]. the antiproliferative capacity of epothilone a in skov-3 cell line (measured as ic50 after 72 h continuous treatment) was six times greater than that of ptx’s. besides, epothilone a induced time coursedependent apoptosis and necrosis [2].
References
[1] regueiro-ren a1, borzilleri rm, zheng x, kim sh, johnson ja, fairchild cr, lee fy, long bh, vite gd. synthesis and biological activity of novel epothilone aziridines. org lett. 2001 aug 23;3(17):2693-6.
[2] rogalska a1, marczak a, gajek a, szwed m, śliwińska a, drzewoski j, jóźwiak z. induction of apoptosis in human ovarian cancer cells by new anticancer compounds, epothilone a and b. toxicol in vitro. 2013 feb;27(1):239-49.
Properties of Epothilone A
| Melting point: | 95℃ |
| Boiling point: | 683.3±55.0 °C(Predicted) |
| alpha | D21 -47.1° (c = 1.0 in methanol) |
| Density | 1.143 |
| storage temp. | -20°C |
| solubility | Chloroform (Slightly), Ethyl Acetate (Slightly) |
| form | solid |
| pka | 13.57±0.70(Predicted) |
| color | White to Off-White |
| Stability: | Stable under recommended storage conditions., Stable Under Recommended Storage C |
Safety information for Epothilone A
Computed Descriptors for Epothilone A
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
![(1S,3S,7S,10R,11S,12S,16R)-3-[(1E)-2-[2-(Aminomethyl)-4-thiazolyl]-1-methylethenyl]-7,11-dihydroxy-8,8,10,12,16-pentamethyl-4,17-dioxabicyclo[14.1.0]heptadecane-5,9-dione](https://img.chemicalbook.in/CAS/GIF/280578-49-6.gif)

You may like
-
 (−)-Epothilone A CAS 152044-53-6View Details
(−)-Epothilone A CAS 152044-53-6View Details
152044-53-6 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1