Epothilone B
Synonym(s):EPO906;EpoB;Patupilone
- CAS NO.:152044-54-7
- Empirical Formula: C27H41NO6S
- Molecular Weight: 507.68
- MDL number: MFCD02101921
- EINECS: 604-822-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-29 17:56:15
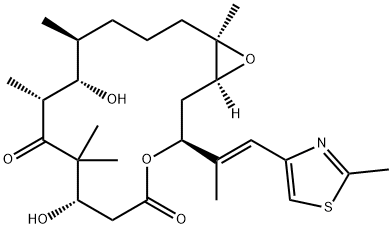
What is Epothilone B?
Description
Epothilone B (152044-54-7) induces microtubule polymerization. Causes cell cycle arrest at the G2-M transition (EC50 = 32 nM for HeLa cells). Induces apoptosis. Cell permeable.
Chemical properties
White Powder
The Uses of Epothilone B
Epothilone B is a microtubule inhibitor isolated from the myxobacteria, Sorangium cellulosum. Like epothilone A, epothilone B acts by stabilising microtubule formation at the taxol binding site, and causes cell cycle arrest at the G2/M transition, leading to cytotoxicity.
The Uses of Epothilone B
Epothilone B (Epo B) is a macrolide that causes the formation of bundles of intracellular microtubules in non-mitotic cells, induces the formation of hyperstable tubulin polymers, and arrests cell cycling in mitosis. It induces mitotic arrest at the G2-M transition in Hs578T and HeLa cells (IC50 = 3 and 32 nM, respectively) as well as in multidrug resistant KB3-1 and KBV-1 cells (IC50 = 16 and 92 nM, respectively). Epo B causes cell cycle arrest at nanomolar IC50 values in cell lines from ovarian, breast, lung, colon, prostate, and squamous cancer.
The Uses of Epothilone B
Epothilones are polyketide natural products that inhibit cancer cells by a mechanism similar to paclitaxel, and also are effective against paclitaxel-resistant tumours. Epothilone B is what is known as a microtubule stabilizer. When a cell divides, the chromosomes that will end up in each cell are separated by thin filaments called microtubules. Normally these microtubules then break down as the cell division progresses. This class of cytotoxic chemo drugs prevents that break down and thus prevents cells from completing division.
Definition
ChEBI: An epithilone that is epithilone D in which the double bond in the macrocyclic ring has been oxidised to the corresponding epoxide (the S,S stereoisomer).
What are the applications of Application
Epothilone B is a microtubule stabilizer
What are the applications of Application
Epothilone B, Synthetic is a potent microtubule stabilizer
General Description
Epothilones has antimitotic properties. It prevents microtubule depolymerization and competitively blocks paclitaxel binding to microtubules. Epothilone B is used to treat metastatic breast cancer (MBC).
Biological Activity
Microtubule stabilization agent that promotes tubulin polymerization and induces G 2 -M cell cycle arrest (EC 50 = 3 - 92 nM). Potently inhibits a variety of human cancer cell lines (IC 50 values are 0.13 - 0.64 nM), including MDR cells overexpressing the P-glycoprotein efflux pump. Exhibits potent anticancer activity in numerous human tumor xenografts in vivo .
Biochem/physiol Actions
(-)-Epothilone B is a microtubule (MT) stabilizing drug and natural macrolide antitumor from myxobacteria Sorangium cellulosum. EpoB has similar biological properties to EpoA. However, EpoB is 10-fold more potent than EpoA against P-glycoprotein-expressing multidrug resistant (MDR) cells (IC50 = 2 nM for MDR CCRF-CEM/VBL100 cells). (-)-Epothilone B is similar to paclitaxel in binding displacement, and a substitution for paclitaxel in dependent cell growth. EpoB causes cell cycle arrest (IC50 = 3.5 nM).
Side Effects
Patupilone is naturally occurring epothilone B. The major dose-limiting adverse reaction to patupilone is diarrhea, rather than neuropathy. Other significant adverse reactions include fatigue and nausea. In phase I/II studies, severe diarrhea was reported in 14–19% of patients. Hematological toxicity appears to be minimal. Grade 3 peripheral neuropathy was the dose-limiting adverse reaction in one phase I study, along with grade 3 diarrhea[3].
References
1) Goodin et al. (2004), Epothilones: mechanism of action and biologic activity; J. Clin. Oncol., 22 2015
2) Bollag et al. (1995), Epothilones, a new class of microtubule-stabilizing agents with a taxol-like mechanism of action; Cancer Res., 55 2325.
3) Lipp, H. and J. Hartmann. “Chapter 45 - Cytostatic and cytotoxic drugs.”Side Effects of Drugs Annual (2011):935-962.
Properties of Epothilone B
| Melting point: | 95-97°C |
| Boiling point: | 680.2±55.0 °C(Predicted) |
| alpha | D21 -35° (c = 0.7 in methanol) |
| Density | 1.136 |
| storage temp. | Store at -20°C |
| solubility | Soluble in DMSO (up to 40 mg/ml) or in Ethanol (up to 35 mg/ml). |
| pka | 13.57±0.70(Predicted) |
| form | White to off-white solid |
| color | colorless |
| Stability: | Stable for 2 years from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20° for up to 1 month. |
| CAS DataBase Reference | 152044-54-7 |
Safety information for Epothilone B
Computed Descriptors for Epothilone B
| InChIKey | QXRSDHAAWVKZLJ-PVYNADRNSA-N |
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
![4-[(1E,3S,5Z,8R/S,10S)-3,11-Bis-{[tert-butyl(dimethyl)silyl]oxy}-2,6,10-trimethyl-8-(phenylsulfonyl)undeca-1,5-dienyl]-2-methyl-1,3-thiazole](https://img.chemicalbook.in/CAS2/GIF/308357-81-5.gif)
![BOC-3-ENDO-AMINOBICYCLO[2.2.1]HEPTANE-2-ENDO-CARBOXYLIC ACID](https://img.chemicalbook.in/StructureFile/ChemBookStructure5/GIF/CB0152129.gif)
![(αR,βS)-β-[[(1,1-DiMethylethoxy)carbonyl]aMino]-α-hydroxy-4-(phenylMethoxy)-benzenepropanoic Acid Ethyl Ester](https://img.chemicalbook.in/CAS/GIF/382596-26-1.gif)

You may like
-
 Epothilone B 152044-54-7 98%View Details
Epothilone B 152044-54-7 98%View Details
152044-54-7 -
 Epothilone B CAS 152044-54-7View Details
Epothilone B CAS 152044-54-7View Details
152044-54-7 -
 (−)-Epothilone B CAS 152044-54-7View Details
(−)-Epothilone B CAS 152044-54-7View Details
152044-54-7 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
