Epirubicin hydrochloride
Synonym(s):4′-Epidoxorubicin hydrochloride;Epidoxorubicin hydrochloride
- CAS NO.:56390-09-1
- Empirical Formula: C27H30ClNO11
- Molecular Weight: 579.9802
- MDL number: MFCD00274554
- EINECS: 260-145-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-02-21 21:32:25
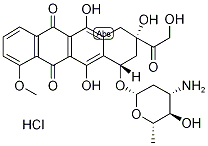
What is Epirubicin hydrochloride?
Description
Epirubicin hydrochloride is an antitumor antibiotic which is epimeric with doxorubicin a t the 4’-position of the amino sugar moiety. It has shown utility in the treatment of mammary, gastric, colorectal, pulmonary and ovarian carcinomas, as well as malignant lymphoma and melanoma and soft tissue sarcoma. It is reported to be less sardiotoxic than doxorubicin.
Description
Epirubicin is a stereoisomer of the antitumor anthracycline doxorubicin that undergoes β-
Chemical properties
Orange-Red Crystalline Solid
Originator
Erbamont (Italy)
The Uses of Epirubicin hydrochloride
Used as an antineoplastic
The Uses of Epirubicin hydrochloride
Cell-permeable anthracycline anti-tumor antibiotic
What are the applications of Application
Epirubicin Hydrochloride is a cell-permeable Topo II inhibitor
brand name
Ellence(Pfizer);FARMORUBICIN.
Biological Functions
This stereoisomer of doxorubicin has the 4′-hydroxy group of the daunosamine sugar oriented in the β-position . Epirubicin will be slowly reduced to the active C13 alcohol (epirubicinol), giving it a 30- to 38-hour half life, which is similar to that of doxorubicin. Unlike doxorubicinol, however, which was equally active with doxorubicin, epirubicinol has only one-tenth the activity of its parent drug and is not believed to contribute significantly to the therapeutic action of this agent.
Biological Activity
Antibiotic antitumor agent. Inhibits the synthesis and function of DNA (IC 50 = 62.7 μ M in rat glioblastoma cell lines) and inhibits the relaxing property of topoisomerase II.
Clinical Use
Epirubicin is indicated for use in breast cancer, and the starting dose is 100 to 120 mg/m2 (compared to a starting dose of 60–75 mg/m2 for doxorubicin).
Side Effects
The side effects and precautions are as outlined previously for doxorubicin, although there is a lower risk of serious myocardial toxicity or myelotoxicity.
Drug interactions
Potentially hazardous interactions with other drugs
Antipsychotics: avoid with clozapine - increased risk
of agranulocytosis.
Ciclosporin: increased risk of neurotoxicity
Cytotoxics: possible increased risk of cardiotoxicity
with trastuzumab - avoid for up to 28 weeks after
stopping trastuzumab.
Ulcer-healing drugs: concentration increased by
cimetidine.
Vaccines: avoid with live vaccines
Metabolism
Cleavage of the epimerized sugar will occur before excretion, generating an aglycone that is indistinguishable from that generated by doxorubicin. Although excretion is primarily biliary, dosage reduction in severe renal impairment, as well as in hepatic dysfunction, is warranted.
storage
+4°C
References
1) Cersosimo?et al. (1986),?Epirubicin: a review of the pharmacology, clinical activity, and adverse effects of an adriamycin analogue; J. Clin. Oncol.,?4?425 2) Spadari?et al. (1986),?DNA polymerases and DNA topoisomerases as targets for the development of anticancer drugs; Anticancer Res.,?6?935 3) Minotti?et al. (2004),?Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity; Pharmacol. Rev.,?56?185 4) Mercuro?et al. (2007),?Early epirubicin-induced myocardial dysfunction revealed by serial tissue Doppler echocardiography: correlation with inflammatory and oxidative stress markers; Oncologist,?12?1124
Properties of Epirubicin hydrochloride
| Melting point: | 185°C dec. |
| alpha | D20 +274° (c = 0.01 in methanol) |
| RTECS | QI9295750 |
| Flash point: | 443.8℃ |
| storage temp. | Inert atmosphere,Store in freezer, under -20°C |
| solubility | Soluble in DMSO to 100mM, or in ethanol to 10mM |
| form | powder |
| color | red to deep red |
| Stability: | Stable for 2 years from date of purchase as supplied. Solutions in DMSO or distilled water may be stored at -20° for up to 3 months. |
Safety information for Epirubicin hydrochloride
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H340:Germ cell mutagenicity H350:Carcinogenicity H360:Reproductive toxicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Epirubicin hydrochloride
| InChIKey | MWWSFMDVAYGXBV-UAOJCOQHSA-N |
| SMILES | C12=C(O)C3=C(C(=O)C4=C(C3=O)C=CC=C4OC)C(O)=C1[C@@]([H])(O[C@H]1O[C@@H](C)[C@H](O)[C@@H](N)C1)C[C@](O)(C(=O)CO)C2.Cl |
Epirubicin hydrochloride manufacturer
Archerchem Healthcare Pvt., Ltd. (part of Archerchem Group)
New Products
1-Boc-4-cyanopiperidine tert-Butyl carbazate 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE TETRABUTYLAMMONIUM CYANIDE TETRAHYDRO-2H-PYRAN-3-OL 3-Pyridineacrylic acid Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% Tadalafil Clopidogrel bisulfate Sitagliptin Phosphate Monohydrate Cabergoline Fexofinadine HCl Etoricoxib 4-Amino Acetophenone 2-Chloro Acetophenone Amlodipine Base 2,3,5-Triiodobenzoic Acid Pyrrolidine Diiodo PentoxideRelated products of tetrahydrofuran

You may like
-
 56390-09-1 Epirubicin hydrochloride 98%View Details
56390-09-1 Epirubicin hydrochloride 98%View Details
56390-09-1 -
 56390-09-1 98%View Details
56390-09-1 98%View Details
56390-09-1 -
 Epirubicin Hydrochloride (EPR.HCl) CAS 56390-09-1View Details
Epirubicin Hydrochloride (EPR.HCl) CAS 56390-09-1View Details
56390-09-1 -
 Epirubicin hydrochloride CAS 56390-09-1View Details
Epirubicin hydrochloride CAS 56390-09-1View Details
56390-09-1 -
 366789-02-8 Riveroxaban 98%View Details
366789-02-8 Riveroxaban 98%View Details
366789-02-8 -
 Carvedilol 98%View Details
Carvedilol 98%View Details
72956-09-3 -
 73590-58-6 Omeprazole 98%View Details
73590-58-6 Omeprazole 98%View Details
73590-58-6 -
 Sertraline HCl 98%View Details
Sertraline HCl 98%View Details
79559-97-0
