Epichlorohydrin
Synonym(s):(±)-2-(Chloromethyl)oxirane;1-Chloro-2,3-epoxypropane
- CAS NO.:106-89-8
- Empirical Formula: C3H5ClO
- Molecular Weight: 92.52
- MDL number: MFCD00005132
- EINECS: 203-439-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-11-28 13:50:20

What is Epichlorohydrin?
Description
Epichlorohydrin, aka 2-(chloromethyl)oxirane, is a hazardous bifunctional liquid with a chloroform-like odor. Insoluble in water; miscible with ethanol, diethyl ether, chloroform, trichloroethylene and carbon tetrachloride; immiscible with petroleum hydro carbons.
Description
Epoxy resins of the bisphenol A type are synthesized from epichlorhydrin and bisphenol A. This leads to bisphenol-A diglycidyl ether, which is the monomer of bisphenol-A-based epoxy resins. Sensitization to epichlorhydrin occurs mainly in workers in the epoxy-resin industry. Sensitization in individuals not working at epoxy resin plants is rare. It has however been described to occur after contact with a soil fumigant, due to solvent cement and in a worker in a pharmaceutical plant, in a division for drug synthesis. Epichlorhydrin was used for the production of both drugs propranolol and oxprenolol.
Chemical properties
Epichlorohydrin is a colorless liquid with a slightly irritating, chloroform-like odor.
Physical properties
Clear, colorless, mobile liquid with a strong, irritating, chloroform-like odor. Odor threshold concentration is 0.93 ppm (quoted, Amoore and Hautala, 1983).
The Uses of Epichlorohydrin
Epichlorohydrin is used to make glycerol,epoxy resins, adhesive, and castings; asderivatives for producing dyes, pharmaceu-ticals, surfactants, and plasticizers; and asa solvent for resins, gums, paints, andvarnishes.
The Uses of Epichlorohydrin
Solvent for natural and synthetic resins, gums, cellulose esters and ethers, paints, varnishes, nail enamels and lacquers, cement for Celluloid. As stabilizer.
The Uses of Epichlorohydrin
Commercially the most important use is production of glycerine. Large volumes are consumed in nonglycerine areas, which largely consist of the various epoxy resins. It has use as a solvent and in the production of epichlorohydrin rubber.
Definition
ChEBI: An epoxide that is 1,2-epoxypropene in which one of the methyl hydrogens is substituted by chlorine.
Production Methods
Epichlorohydrin can be prepared from 1,3-dichloropropanol-2, 2,3- dichloropropanol-1, or allyl chloride. Commercially it is prepared as an intermediate in glycerol synthesis via alkaline hydrolysis of glycerol dichlorohydrin. Both come from allyl chloride. Epichlorohydrin reacts with monohydric alcohols to give ethers by opening the oxide ring. It will react with ethers, aldehydes, ketones, organic acids and amines to give a wide variety of useful syntheses.
Preparation
Epichlorohydrin is traditionally manufactured from allyl chloride in two steps, beginning with the addition of hypochlorous acid, which affords a mixture of two isomeric alcohols:
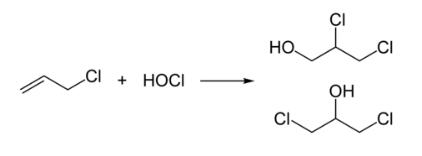
In the second step, this mixture is treated with base to give the epoxide:
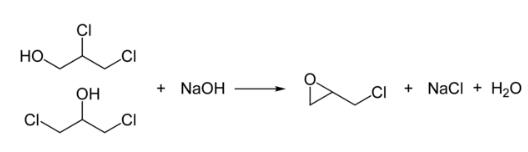
Health effects
Epichlorohydrin is a PROBABLE CARCINOGEN in humans. There is evidence that it causes lung cancer in humans and it has been shown to cause nasal cavity and skin cancer in animals.
Synthesis Reference(s)
Organic Syntheses, Coll. Vol. 2, p. 256, 1943
The Journal of Organic Chemistry, 48, p. 3831, 1983 DOI: 10.1021/jo00169a052
General Description
A clear colorless liquid with an irritating chloroform-like odor. Density 9.8 lb / gal. Flash point 87°F. Polymerizable. If polymerization takes place inside a closed container, the container is subject to violent rupture. Irritates the skin and respiratory system. Toxic by ingestion. A confirmed carcinogen. Vapors heavier than air. Used to make plastics and as a solvent.
Air & Water Reactions
Highly flammable. Water soluble.
Reactivity Profile
Epichlorohydrin may polymerize exothermically if heated or contaminated. Reacts explosively with aniline. Ignites on contact with potassium tert-butoxide. Reacts with trichloroethylene to give the explosive dichloroacetylene. Violent reaction with sulfuric acid or isopropylamine. Exothermic polymerization on contact with strong acids or bases, zinc, aluminum, aluminum chloride, iron, ferric chloride [Sax, 9th ed., 1996, p. 1469].
Hazard
Toxic by inhalation, ingestion, and skin absorption; strong irritant, a carcinogen. Flammable, moderate fire risk. TLV: 0.5 ppm; animal carcinogen.
Health Hazard
Epichlorohydrin is caustic as both a liquid and gas. Irritation of the eyes and skin, and skin sensitization has been observed. Exposure to epichlorohydrin has caused inflammation of the lungs, asthmatic bronchitis, and liver and kidney damage. In acute poisonings, death may be caused by respiratory paralysis.
Health Hazard
Epichlorohydrin is toxic, carcinogenic, and astrong irritant. Its vapors can produce irrita-tion in the eyes, skin, and respiratory tract.Exposure to high concentration resulted indeath in animals, injuring the central nervoussystem. The liquid can absorb through humanskin, causing painful irritation of subcuta-neous tissues (ACGIH 1986). The symptomsof toxicity from high dosage in test animalswere paralysis of muscles and slow devel-opment of respiratory distress. Long expo-sures at 120 ppm for several hours resultedin lung, kidney, and liver injury in rats(Gage 1959). Ingestion by an oral routecaused tremor, somnolence, and ataxia inmice (NIOSH 1986). The toxic symptomsand lethal doses varied widely with animalspecies. The toxic metabolite of epichlorhy-drin could be ?- chlorohydrin ; thelatter was produced in vitro by rat livermicrosomes (Gingell et al. 1987).
A 25 ppm concentration may be detectableby odor. Exposure at this level may causeburning of the eyes and nose in humans.Above 100 ppm even a short exposure maybe hazardous to humans, causing nausea,dyspnea, lung edema, and kidney injury.
Epichlorohydrin is mutagenic and hasshown carcinogenicity in test animals. Itcaused tumors in the lungs and nose andat gastrointestinal and endocrine sites. Expo-sure to this compound caused harmful repro-ductive effects on fertility and birth defectsin mice.
Fire Hazard
When heated to decomposition, Epichlorohydrin evolves highly toxic fumes of phosgene and carbon monoxide. Reactive and incompatible with strong oxidizers, strong acids, caustics, zinc, aluminum, chlorides of iron and aluminumand compounds with an active hydrogen atom, including water. Unstable, avoid heat, contaminants, strong acids and bases, certain curing agents such as ethylenediamine. Hazardous polymerization may occur.
Flammability and Explosibility
Flammable
Chemical Reactivity
Reactivity with Water Mild reaction; not likely to be hazardous; Reactivity with Common Materials: No reaction; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Can polymerize in presence of strong acids and bases, particularly when hot; Inhibitor of Polymerization: None used.
Contact allergens
Epoxy resin of the Bisphenol A type is synthesized from epichlorhydrin and bisphenol A. It leads to bisphenol A diglycidyl ether, which is the monomer ofbisphenol-A-based epoxy resins. Sensitization to epichlorhydrin occurs mainly in workers of the epoxy resin industry. Sensitization in individuals not working at epoxy resin plants is rare. It has, however, been described to occur following exposure to a soil fumigant, due to solvent cement, and in a worker in a pharmaceutical plant, in a division of drug synthesis. Epichlorhydrin was used for the production of drugs propranolol and oxprenolol.
Safety Profile
Confirmed carcinogen with experimental carcinogenic data. Poison by ingestion, skin contact, intravenous, and intraperitoneal routes. Moderately toxic by inhalation. An experimental teratogen. Other experimental reproductive effects. Human systemic effects by inhalation: respiratory, nose, and eyes. Human mutation data reported. A skin and eye irritant. A sensitizer. Flammable liquid when exposed to heat or flame. Explosive reaction with andine. Reaction with trichloroethylene forms the explosive dichloroacetylene. Ignition on contact with potassium tertbutoxide. Violent reaction with sulfuric acid or isopropylamine. Exothermic polymerization on contact with strong acids, caustic alkalies, aluminum, aluminum chloride, iron(II1) chloride, or zinc. When heated to decomposition it emits toxic fumes of Cl
Potential Exposure
Epichlorohydrin, an organochlorine, is used in the manufacture of many glycerol and glycidol derivatives and epoxy resins; as a stabilizer in chlorine-containing materials; as an intermediate in the preparation of cellulose esters and ethers, paints, varnishes, nail enamels, and lacquers; as a cement for celluloid. It is used as an intermediate in the manufacture of various drugs. Increased cancer risk.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.Medical observation is recommended for 24-48 h afterbreathing overexposure, as pulmonary edema may bedelayed. As first aid for pulmonary edema, a doctor orauthorized paramedic may consider administering a corticosteroid spray
Carcinogenicity
Epichlorohydrin is reasonably anticipated to be a human carcinogenbased on sufficient evidence of carcinogenicity from studies in experimental animals.
Environmental Fate
Biological. Bridié et al. (1979) reported BOD and COD values of 0.03 and 1.16 g/g using
filtered effluent from a biological sanitary waste treatment plant. These values were determined
using a standard dilution method at 20 °C for a period of 5 d. When a sewage seed was used in a
separate screening test, a BOD value of 0.16 g/g was obtained. The ThOD for epichlorohydrin is
1.21 g/g.
Chemical/Physical. Anticipated products from the reaction of epichlorohydrin with ozone or
OH radicals in the atmosphere are formaldehyde, glyoxylic acid, and ClCH2O(O)OHCHO (Cupitt,
1980). Haag and Yao (1992) reported a calculated OH radical rate constant in water of 2.9 x
108/M?sec.
Storage
Epichlorohydrin is stored in a well-ventilated,cool place isolated from combustible andoxidizable materials, all acids and bases,and anhydrous metal halides. Protect fromphysical damage. It is shipped in metaldrums.
Shipping
UN2023 Epichlorhydrin, Hazard class: 6.1; Labels: 6.1-Poisonous materials, 3-Flammable liquid.
Purification Methods
Distil epichlorohydrin under atmospheric pressure, heat it on a steam bath with one-quarter its weight of CaO, then decant and fractionally distil it. [Beilstein 17 V 20.]
Toxicity evaluation
Epichlorohydrin is an alkylating agent that is mutagenic. It may induce DNA interstrand cross-links, chromosomal aberrations, and breaks. It is also an irritant, sensitizer, and corrosive.
Incompatibilities
May form explosive mixture with air. Slowly decomposes on contact with water. Heat or strong acids; alkalies, metallic halides, or contaminants can cause explosive polymerization. Violent reaction with strong oxidizers, aliphatic amines; alkanolamines, amines (especially aniline), alkaline earths; chemically active metals (chlorides of aluminum, iron zinc); powdered metals (aluminum, zinc); alcohols, phenols, organic acids; causing fire and explosion hazard. Will pit steel in the presence of water. Thermal decomposition forms highly toxic phosgene gas. May accumulate static electrical charges, and may cause ignition of its vapors.
Waste Disposal
Incineration, preferably after mixing with another combustible fuel. Care must be exercised to assure complete combustion to prevent the formation of phosgene. An acid scrubber is necessary to remove the halo acids produced. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≧100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal
Properties of Epichlorohydrin
| Melting point: | -57 °C |
| Boiling point: | 115-117 °C(lit.) |
| alpha | -1~+1°(D/20℃)(c=1,CH3OH) |
| Density | 1.183 g/mL at 25 °C(lit.) |
| vapor density | 3.2 (vs air) |
| vapor pressure | 13.8 mm Hg ( 21.1 °C) |
| refractive index | n |
| Flash point: | 93 °F |
| storage temp. | Store below +30°C. |
| solubility | 65.9g/l |
| appearance | colorless liquid |
| form | Liquid |
| color | APHA: ≤20 |
| Specific Gravity | 1.183 (20/4℃) |
| Odor | Pungent, garlic; sweet, pungent; like chloroform. |
| explosive limit | 3.8-21%(V) |
| Water Solubility | 6 g/100 mL (10 ºC) |
| FreezingPoint | -57.2℃ |
| Merck | 14,3611 |
| BRN | 79785 |
| Henry's Law Constant | 3.42(x 10-5 atm?m3/mol) at 25 °C (static headspace-GC, Welke et al., 1998) |
| Exposure limits | TLV-TWA(skin) 8 mg/m3
(2 ppm) (ACGIH);
STEL (15 min) 19 mg/m3
(5 ppm) (NIOSH). |
| Dielectric constant | 22.9(20℃) |
| Stability: | Unstable. Flammable - note wide explosion limits and low flash point. |
| CAS DataBase Reference | 106-89-8(CAS DataBase Reference) |
| IARC | 2A (Vol. 11, Sup 7, 71) 1999 |
| NIST Chemistry Reference | Oxirane, (chloromethyl)-(106-89-8) |
| EPA Substance Registry System | Epichlorohydrin (106-89-8) |
Safety information for Epichlorohydrin
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H226:Flammable liquids H314:Skin corrosion/irritation H317:Sensitisation, Skin H350:Carcinogenicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Epichlorohydrin
Epichlorohydrin manufacturer
PS Polychem
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran



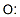
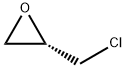
You may like
-
 Epichlorohydrin 99%View Details
Epichlorohydrin 99%View Details -
 Epichlorohydrin 98%View Details
Epichlorohydrin 98%View Details -
 Epichlorohydrin CAS 106-89-8View Details
Epichlorohydrin CAS 106-89-8View Details
106-89-8 -
 Epichlorohydrin extrapure AR CAS 106-89-8View Details
Epichlorohydrin extrapure AR CAS 106-89-8View Details
106-89-8 -
 Epichlorohydrin CASView Details
Epichlorohydrin CASView Details -
 Epichlorohydrin, Grade: Industrial, Purity: 99View Details
Epichlorohydrin, Grade: Industrial, Purity: 99View Details
106-89-8 -
 Epichlorohydrin Liquid Chemical, Grade: IndustrialView Details
Epichlorohydrin Liquid Chemical, Grade: IndustrialView Details
106-89-8 -
 Liquid Epichlorohydrin Chemical, Packaging Size: 172 Kg, Packaging Type: DrumView Details
Liquid Epichlorohydrin Chemical, Packaging Size: 172 Kg, Packaging Type: DrumView Details
106-89-8
