Trelagliptin succinate
- CAS NO.:1029877-94-8
- Empirical Formula: C22H26FN5O6
- Molecular Weight: 475.48
- MDL number: MFCD22665720
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 16:58:18
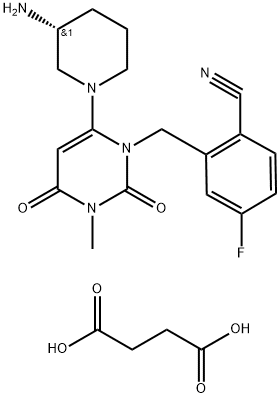
What is Trelagliptin succinate?
Description
Similar to omarigliptin, trelagliptin succinate (XIX) is a highly selective, orally delivered inhibitor of DPP-4 developed by Takeda Pharmaceuticals and approved in Japan in March 2015 for the treatment of type 2 DM. Interestingly, trelagliptin is structurally similar to alogliptin, a DPP-4 inhibitor also marketed by Takeda and described in our 2010 review, differing only in the presence of a fluorine in the 5-position of the cyanobenzyl moiety. Both trelagliptin and alogliptin are potent inhibitors of DPP-4, with IC50s of 1.3 and 5.3 nM, respectively. Notably, while similar drugs are dosed once daily, trelagliptin is the first DPP-4 inhibitor approved for onceweekly dosing. Kinetic analysis has revealed that trelagliptin is a substrate-competitive, reversible, slow-binding inhibitor (t1/2 for dissociation = ca. 30 min) of DPP-4, although the dissociation time is insufficient to explain its long-acting effects. In a phase III trial, once-weekly trelagliptin (100 mg) showed similar efficacy and safety to once-daily alogliptin (25 mg) in patients with type 2 DM inadequately controlled by diet and exercise. The medicinal chemistry discovery of trelagliptin and alogliptin as well as reviews of this class of compounds have been published.
The Uses of Trelagliptin succinate
Trelagliptin succinate (SYR-472) is a selective, long acting dipeptidyl peptidase-4 (DPP-4) inhibitor. An antidiabetic agent.
Orally active DPP-4 inhibitor that produces clinically and statistically significant improvements in glycaemic control in patients with type 2 diabetes. SYR472 has a long duration of action and is well tolerated in clinical studies.
Clinical Use
Trelagliptin (SYR-472), a novel dipeptidyl peptidase-4 inhibitor used for the treatment of type 2 diabetes mellitus. Trelagliptin (as the salt Trelagliptin succinate) was approved for use in Japan in March 2015. Takeda, the company that developed Trelagliptin, chose to not get approval for the drug in the USA and EU.
Synthesis
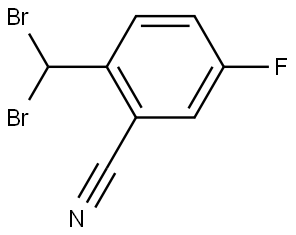
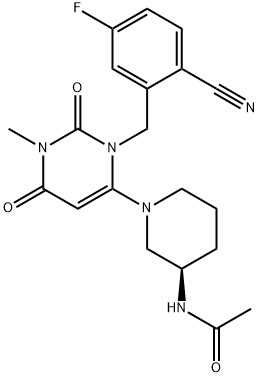
The kilogram-scale synthesis of trelagliptin succinate has been reported in five steps from commercial starting material.102 Commercial 2-bromo-5- fluorotoluene (162) was reacted with copper cyanide in refluxing DMF to provide the corresponding nitrile in 60% yield. Benzylic bromination with AIBN and 1,3-dibromo-5,5- dimethylhydantoin (DBDMH) in DCE followed by treatment with diethyl phosphite and DIPEA gave crude benzyl bromide 163, which was substituted directly with 6-chloro-3-methyluracil (164) in the presence of DIPEA in NMP to provide chloride 165 in 86% yield. Reaction with commercial (R)-3- aminopiperidine dihydrochloride 166 in the presence of K2CO3 and i-PrOH furnished trelagliptin as the freebase. Conversion to the HCl salt and purification by crystallization from dichloromethane, followed by a freebasing via 50% NaOH, and treatment with succinic acid in THF/i-PrOH at 60 ??C, and final recrystallization, generated trelagliptin succinate XIX.
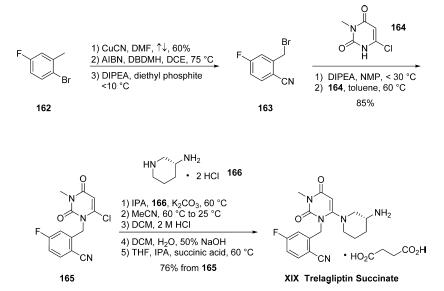
Research
Trelagliptin (Zafatek) is an orally active DPP-4 inhibitor developed by Takeda and approved in Japan for the treatment of type 2 diabetes mellitus (T2DM). Unlike other approved agents of its class, which are usually administered once daily, trelagliptin can be administered once weekly. Phase II development of trelagliptin was discontinued in the USA and EU, as Takeda considered that the costs associated with obtaining approval in these markets were prohibitive.
Properties of Trelagliptin succinate
| storage temp. | -20°C |
| solubility | Soluble in DMSO |
| form | Powder |
| color | Off-white solid |
Safety information for Trelagliptin succinate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Trelagliptin succinate
Trelagliptin succinate manufacturer
Chemzone Pharma
Archerchem Healthcare Pvt., Ltd. (part of Archerchem Group)
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 1029877-94-8 Trelagliptin succinate 98%View Details
1029877-94-8 Trelagliptin succinate 98%View Details
1029877-94-8 -
 1029877-94-8 99%View Details
1029877-94-8 99%View Details
1029877-94-8 -
 Trelagliptin succinate API 98%View Details
Trelagliptin succinate API 98%View Details
1029877-94-8 -
 Trelagliptin succinate 1029877-94-8 / 1186045-73-7 98%View Details
Trelagliptin succinate 1029877-94-8 / 1186045-73-7 98%View Details
1029877-94-8 / 1186045-73-7 -
 1029877-94-8 / 1186045-73-7 Trelagliptin succinate 99%View Details
1029877-94-8 / 1186045-73-7 Trelagliptin succinate 99%View Details
1029877-94-8 / 1186045-73-7 -
 Trelagliptin succinate 95% CAS 1029877-94-8View Details
Trelagliptin succinate 95% CAS 1029877-94-8View Details
1029877-94-8 -
 Pharma Trelagliptin Succinate Cas 1029877 94 8, For Commerical, 99%View Details
Pharma Trelagliptin Succinate Cas 1029877 94 8, For Commerical, 99%View Details
1029877-94-8 -
 Trelagliptin Succinate CAS NO: 1029877-94-8 SPIL, USPView Details
Trelagliptin Succinate CAS NO: 1029877-94-8 SPIL, USPView Details
1029877-94-8
