Entrectinib
- CAS NO.:1108743-60-7
- Empirical Formula: C31H34F2N6O2
- Molecular Weight: 560.64
- MDL number: MFCD28129099
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 15:53:33
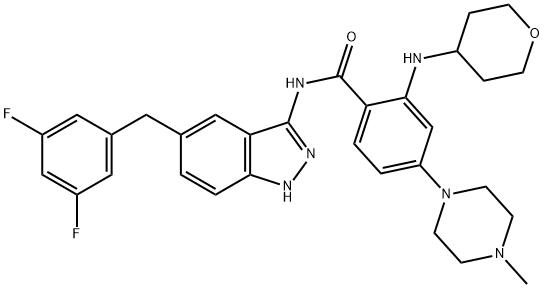
What is Entrectinib?
Absorption
Entrectinib has a Tmax of 4-5 h after administration of a single 600 mg dose. Food does not produce a significant effect on the extent of absorption.
Description
Entrectinib (RXDX-101, Ignyta Pharmaceuticals, San Diego, CA, USA) is a small molecule that inhibits the tyrosine kinases TRKA/B/C, ROS1, and ALK (Table?1). It has a preclinical median inhibitory concentration (IC50) of 7?nm against ROS1, higher than crizotinib [95, 96]. Entrectinib was specifically designed to cross the blood-brain barrier [95].
Description
Entrectinib is an inhibitor of TrkA (IC50 = 1.7 nM), TrkB (IC50 = 0.1 nM), and TrkC (IC50 = 0.1 nM), as well as C-ros oncogene 1 (ROS1; IC50 = 0.2 nM) and anaplastic lymphoma kinase (ALK; IC50 = 1.6 nM). Entrectinib blocks proliferation of ALK-dependent cell lines, including those with L1196M or C1156Y resistance mutations, and inhibits ALK‐dependent signaling. It has been shown to inhibit the growth of a non-small cell lung cancer cell line bearing an EML4-ALK rearrangement. In mice bearing various Trk, ROS1, or ALK-driven xenografts, entrectinib has been shown to induce tumor regression.
The Uses of Entrectinib
Entrectinib, also known as RXDX-101 and NMS-E628, is an oral small molecule inhibitor of TrkA, TrkB and TrkC, as well as ROS1 and ALK, with high potency and selectivity. RXDX-101 has demonstrated potent pharmacological activity in preclinical studies and has the potential to be first-in-class against the Trk family of kinases. PXDX-101 has been well tolerated in patients with advanced solid tumors. PXDX-101 is currently in clinical trials, and is being developed by Ignyta.
Indications
Entrectinib is indicated for the treatment of metastatic ROS1-positive non-small cell lung cancer in adults. Entrectinib is also indicated in adults and children over 12 years old for the treatment of NTRK gene fusion-positive solid tumors which have metastasized or for which surgical resection is likely to result in severe morbidity and for which has progressed on previous therapies or for which no comparable alternative therapies are available.
FoundationOne?Liquid CDx is the only FDA-approved test for the detection of ROS1 rearrangement(s) in NSCLC for selecting patients for treatment with entrectinib.
Background
Entrectinib is a tropomyosin receptor tyrosine kinase (TRK) TRKA, TRKB, TRKC, proto-oncogene tyrosine-protein kinase ROS1, and anaplastic lymphoma kinase (ALK) inhibitor. It was approved by the FDA in August 2019 for use in the treatment of ROS1-positive metastatic non-small cell lung cancer and NTRK gene fusion positive solid tumors. Entrectinib's approved use is meant as a last line of therapy due to its accelerated approval based on early trial data. This therapy offers benefit over similar ALK inhibitors such as alectinib, ceritinib, and lorlatinib due to a wider range of targets.
brand name
Rozlytrek
General Description
Class: receptor tyrosine kinase; Treatment: NTRK tumors, ROS-1 NSCLC; Other name: RXDX-101; Elimination half-life = 20 h; Protein binding >99%
Pharmacokinetics
Entrectinib and its active metabolite suppress several pathways which contribute to cell survival and proliferation. This suppression shifts the balance in favor of apoptosis thereby preventing cancer cell growth and shrinking tumors.
Clinical Use
Entrectinib demonstrated potent antitumor effects in tumor cell lines and patient-derived xenograft (PDX) tumor models in preclinical studies. Furthermore, entrectinib can cross the blood–brain barrier (BBB) to impact primary brain tumors and brain metastases in patients with NTRK1/ NTRK2/NTRK3, ROS1, and ALK fusion-driven cancers.
in vitro
entrectinib potently and selectively inhibits the in vitro growth of alk-driven tumors, with confirmed mechanism of action [1].
in vivo
since entrectinib is able to pass the blood-brain barrier, the compound was also tested for efficacy in an xenograft model with alk positive nsclc tumors. mri imaging demonstrated that entrectinib was able to effectively and control the growth of these intracranial tumors dose-dependently, leading to increased survival [1].
Metabolism
CYP3A4 is responsible for 76% of entrectinib metabolism in humans including metabolism to the active metabolite, M5. M5 has similar pharmacological activity to entrectinib and exists at approximately 40% of the steady state concentration of the parent drug. In rats, six in vivo metabolites have been identified including N-dealkylated, N-oxide, hydroxylated, and glucuronide conjugated metabolites.
References
[1] elena ardini, maria menichincheri, patrizia banfi, daniele casero, m. laura giorgini, m. beatrice saccardo, nadia amboldi, nilla avanzi, paolo orsini, antonella isacchi, enrico pesenti, arturo galvani. the alk inhibitor nms-e628 also potently inhibits ros1 and induces tumor regression in ros-driven models. [abstract]. in: proceedings of the 104th annual meeting of the american association for cancer research; 2013 apr 6-10; washington, dc. philadelphia (pa): aacr; cancer res 2013;73(8 suppl):abstract nr 2092. doi:10.1158/1538-7445.am2013-2092
Properties of Entrectinib
| Boiling point: | 717.5±60.0 °C(Predicted) |
| Density | 1.340±0.06 g/cm3(Predicted) |
| storage temp. | Store at -20°C |
| solubility | ≥28.05 mg/mL in DMSO; insoluble in H2O; ≥9.82 mg/mL in EtOH with ultrasonic |
| form | solid |
| pka | 12.01±0.43(Predicted) |
| color | Off-white to light yellow |
Safety information for Entrectinib
Computed Descriptors for Entrectinib
| InChIKey | HAYYBYPASCDWEQ-UHFFFAOYSA-N |
| SMILES | C(NC1C2=C(NN=1)C=CC(CC1=CC(F)=CC(F)=C1)=C2)(=O)C1=CC=C(N2CCN(C)CC2)C=C1NC1CCOCC1 |
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 1108743-60-7 Entrectinib 98%View Details
1108743-60-7 Entrectinib 98%View Details
1108743-60-7 -
 Entrectinib 95% CAS 1108743-60-7View Details
Entrectinib 95% CAS 1108743-60-7View Details
1108743-60-7 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1