ENNIATIN B
Synonym(s):
- CAS NO.:917-13-5
- Empirical Formula: C33H57N3O9
- Molecular Weight: 639.83
- MDL number: MFCD00236425
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-10-28 23:16:16
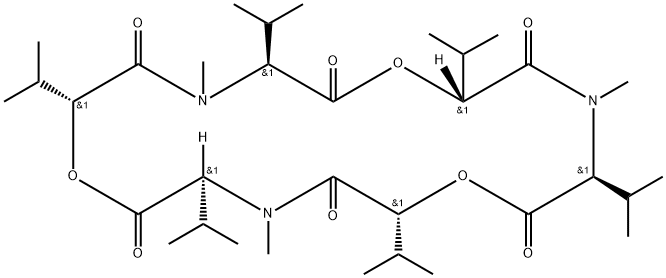
What is ENNIATIN B?
The Uses of ENNIATIN B
Enniatins are a family of depsipeptide ionophores, produced several Fusarium species. More recently, the effects of the ennitins on acyl-CoA cholesterol transferase, transporters and the selectivity of their antitumour action have received more focus. Enniatin B is the most studied of four major analogues of the enniatin complex.
The Uses of ENNIATIN B
Enniatins are a family of depsipeptide ionophores produced by several Fusarium species. Recently, the effects of the enniatins on acyl-CoA cholesterol transferase, transporters and the selectivity of their antitumor action have received more focus. Enniatin B is the most studied of four major analogues of the enniatin complex.
What are the applications of Application
Enniatin B is an ionophore antibiotic that inhibits ACAT and PDE
Definition
ChEBI: An enniatin obtained from formal cyclocondensation of three N-[(2R)-2-hydroxy-3-methylbutanoyl]-N-methyl-L-valine units.
Biological Activity
enniatin b is an ionophore antibiotic.enniatins are one of the cyclohexadepsipeptides produced by various species of the genus fusarium, and are reported to have antibiotic, ionophoric, and in-vitro hypolipidaemic activities.
Biochem/physiol Actions
Enniatins are a group of cyclohexadepsipeptide mycotoxins produced by Gnomonia errabuda and several Fusaria species, with phytotoxic, antibiotic, and insecticidal activities. Enniatins function as ionophors by their incorporation into the cellular membrane to form dimeric structures that transport monovalent ions across the membrane (especially the mitochondrial membranes) affecting oxidative phosphorylation uncoupling. It has been demonstrated that enniatins have a cytotoxic effect on human cancer cells. Furthermore, incubation of H4IIE hepatoma cells with enniatins strongly diminished phosphorylation of the ERK (p44/p42). Enniatins B and B1 inhibit the multi-drug resistance transporter Pdr5p from Saccharomyces cerevisiae, indicating their beneficial potential in cases of drug resistant patients.
in vitro
a non-toxic concentration of enniatin b could strongly inhibit a pdr5p-mediated efflux of cycloheximide or cerulenin in pdr5p-overexpressing cells. the mode of pdr5p inhibition caused by enniatin b was competitive against fk506. however, enniatin b could not inhibit the function of snq2p, a homologue of pdr5p [1]. another study showed that enniatin b was a relatively poor ionophore that could facilitate import of k+ and na+ across membranes [2]. it was also found that like other enniatins, enniatin b was able to inhibit acyl-coa: cholesterol acyltransferase [3].
in vivo
after oral administration to mice, no toxicological signs or pathological changes were observed. moreover, enniatin b was found in all tissues and serum but not in urine, and the highest amounts was measured in liver and fat. three phase i metabolites of enniatin b were found in liver and colon, with dioxygenated-enniatin b being most prominent [4].
References
[1] k. hiraga, s. yamamoto, h. fukuda, et al. enniatin has a new function as an inhibitor of pdr5p, one of the abc transporters in saccharomyces cerevisiae. biochemical and biophysical research communications 328(4), 1119-1125 (2005).
[2] m. r. kamyar, p. rawnduzi, c. r. studenik, et al. investigation of the electrophysiological properties of enniatins. archives of biochemistry and biophysics429(2), 215-223 (2004).
[3] tomoda, x. h. huang, j. cao, et al. inhibition of acyl-coa: cholesterol acyltransferase activity by cyclodepsipeptide antibiotics. j.antibiot.(tokyo) 45(10),1626-1632 (1992).
[4] rodríguez-carrasco y et al. mouse tissue distribution and persistence of the food-born fusariotoxins enniatin b and beauvericin. toxicol lett. 2016 apr 15;247:35-44.
Properties of ENNIATIN B
| storage temp. | -20°C |
| solubility | DMSO: soluble10mg/mL |
| form | White to off-white crystalline solid. |
| Boiling point: | 827.0±65.0 °C(Predicted) |
| Density | 1.036±0.06 g/cm3(Predicted) |
| Melting point: | 229 °C |
| pka | -1.08±0.70(Predicted) |
Safety information for ENNIATIN B
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
Computed Descriptors for ENNIATIN B
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 Enniatin B CAS 917-13-5View Details
Enniatin B CAS 917-13-5View Details
917-13-5 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1