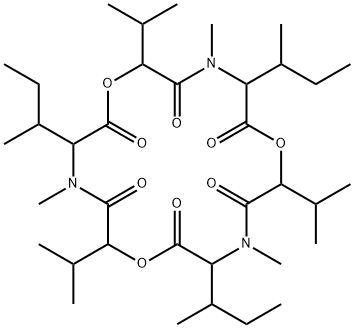
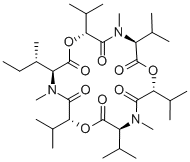
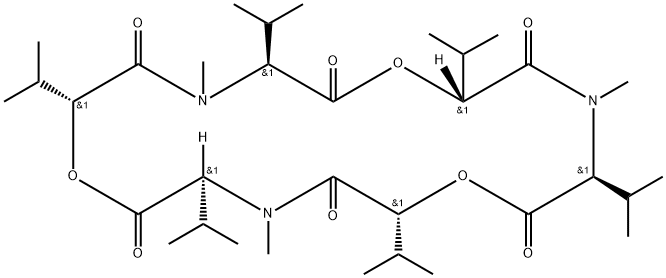
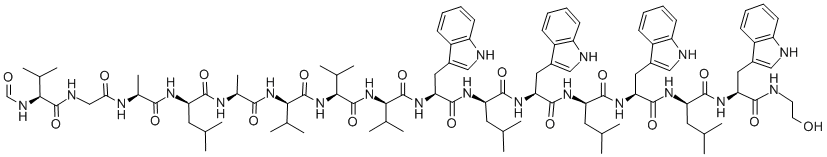
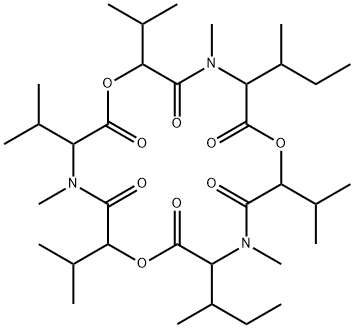
enniatin A1
Synonym(s):2-(N-Methyl-L-valine) Enniatin A;Cyclo[(2R)-2-hydroxy-3-methylbutanoyl-N-methyl-L-isoleucyl-(2R)-2-hydroxy-3-methylbutanoyl-N-methyl-L-isoleucyl-(2R)-2-hydroxy-3-methylbutanoyl-N-methyl-L-valyl]
- CAS NO.:4530-21-6
- Empirical Formula: C35H61N3O9
- Molecular Weight: 667.879
- MDL number: MFCD14635385
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-10-28 23:16:16
What is enniatin A1?
The Uses of enniatin A1
Enniatins are a family of depsipeptide ionophores, produced several Fusarium species. More recently, the effects of the ennitins on acyl-CoA cholesterol transferase, transporters and the selectivity of their antitumour action have received more focus. Enniatin A1 is one of four major analogues of the enniatin complex and has previously not been available for investigation.
The Uses of enniatin A1
Enniatins are a family of depsipeptide ionophores produced by several Fusarium species. Recently, the effects of the enniatins on acyl-CoA cholesterol transferase, transporters and the selectivity of their antitumor action have received more focus. Enniatin A1 is one of four major analogues of the enniatin complex .
The Uses of enniatin A1
Enniatin A1 is a mycotoxin capable of creating negative CNS effects due to their ability to cross the blod-brain barrier. Secondary metabolite of microbes, it is associated with mold, moisture damage and asthma development.
What are the applications of Application
Enniatin A1 is a Fusarium sp. derived enniatin
Definition
ChEBI: An enniatin obtained from formal cyclocondensation of one N-[(2R)-2-hydroxy-3-methylbutanoyl]-N-methyl-L-valine and two N-[(2R)-2-hydroxy-3-methylbu anoyl]-N-methyl-L-isoleucine units.
Biochem/physiol Actions
Enniatins are a group of cyclohexadepsipeptide mycotoxins produced by Gnomonia errabuda and several Fusaria species, with phytotoxic, antibiotic, and insecticidal activities. Enniatins function as ionophors by their incorporation into the cellular membrane to form dimeric structures that transport monovalent ions across the membrane (especially the mitochondrial membranes) affecting oxidative phosphorylation uncoupling. It has been demonstrated that enniatins have a cytotoxic effect on human cancer cells. Furthermore, incubation of H4IIE hepatoma cells with enniatins strongly diminished phosphorylation of the ERK (p44/p42).
Properties of enniatin A1
| Melting point: | 116-117 °C |
| Boiling point: | 840.5±65.0 °C(Predicted) |
| Density | 1.027±0.06 g/cm3(Predicted) |
| storage temp. | -20°C |
| solubility | DMSO: soluble10mg/mL |
| form | White to off-white crystalline solid. |
| pka | -1.44±0.70(Predicted) |
Safety information for enniatin A1
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for enniatin A1
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran
You may like
-
 Enniatin A1 CAS 4530-21-6View Details
Enniatin A1 CAS 4530-21-6View Details
4530-21-6 -
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4