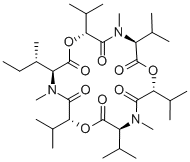
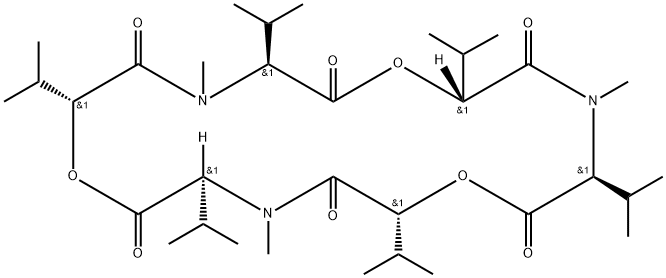
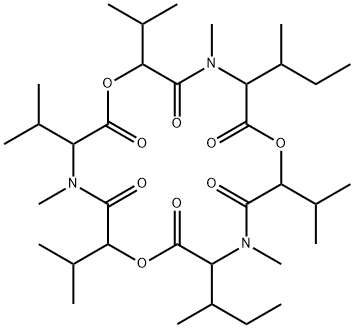
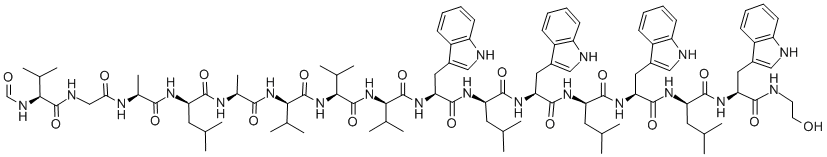
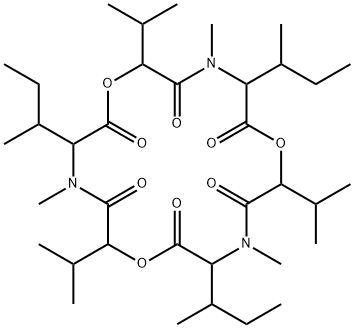
enniatin A
Synonym(s):Cyclo[(2R)- 2-hydroxy-3-methylbutanoyl-N-methyl-L-isoleucyl-(2R)-2-hydroxy-3-methylbutanoyl-N-methyl-L-isoleucyl-(2R)-2-hydroxy-3-methylbutanoyl- N-methyl-L- isoleucyl]
- CAS NO.:2503-13-1
- Empirical Formula: C36H63N3O9
- Molecular Weight: 681.906
- MDL number: MFCD19442801
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-10-28 23:16:16
What is enniatin A?
Description
Enniatins are cyclohexadepsipeptides commonly isolated from fungi that are known to have antibiotic properties and to induce apoptosis in several cancer lines. Many function as ionophores, forming pores in cellular membranes to allow selective ion transport. Enniatin A is one of four major analogs in the enniatin complex . It has been shown to moderately inhibit acyl-CoA:cholesterol acyltranferase activity in rat liver microsomes with an IC50 value of 22 μM. Enniatin A also demonstrates anthelmintic properties against N. brasiliensis, T. spiralis, and H. spumosa.
The Uses of enniatin A
Enniatins are a family of depsipeptide ionophores, produced by several Fusarium species. More recently, the effects of the enniatins on acyl-CoA cholesterol transferase, transporters and the selectivity of their antitumour action have received more focus. Enniatin A is one of four major analogues of the enniatin complex and has not previously been available for investigation.
The Uses of enniatin A
Enniatins are a family of depsipeptide ionophores, produced by several Fusarium species. Recently, the effects of the enniatins on acyl-CoA cholesterol transferase, transporters and the selectivity of their antitumor action have received more focus. Enniatin A is one of four major analogues of the enniatin complex.
The Uses of enniatin A
Enniatins are cyclohexadepsipeptides commonly isolated from fungi that are known to have antibiotic properties and to induce apoptosis in several cancer lines. Many function as ionophores, forming pores in cellular membranes to allow selective ion transport. Enniatin A is one of four major analogs in the enniatin complex . It has been shown to moderately inhibit acyl-CoA:cholesterol acyltranferase activity in rat liver microsomes with an IC50 value of 22 μM. Enniatin A also demonstrates anthelmintic properties against N. brasiliensis, T. spiralis, and H. spumosa.[Cayman Chemical]
What are the applications of Application
Enniatin A is a Fusarium produced depsipeptide ionophore
Definition
ChEBI: An enniatin obtained from formal cyclocondensation of three N-[(2R)-2-hydroxy-3-methylbutanoyl]-N-methyl-L-isoleucine units.
Biochem/physiol Actions
Enniatins are a group of cyclohexadepsipeptide mycotoxins produced by Gnomonia errabuda and several Fusaria species, with phytotoxic, antibiotic, and insecticidal activities. Enniatins function as ionophors by their incorporation into the cellular membrane to form dimeric structures that transport monovalent ions across the membrane (especially the mitochondrial membranes) affecting oxidative phosphorylation uncoupling. It has been demonstrated that enniatins have a cytotoxic effect on human cancer cells. Furthermore, incubation of H4IIE hepatoma cells with enniatins strongly diminished phosphorylation of the ERK (p44/p42).
Properties of enniatin A
| Melting point: | 122-124 °C |
| Boiling point: | 847.4±65.0 °C(Predicted) |
| Density | 1.022±0.06 g/cm3(Predicted) |
| storage temp. | -20°C |
| solubility | DMSO: soluble10mg/mL |
| form | White to off-white crystalline solid. |
| pka | -0.96±0.70(Predicted) |
Safety information for enniatin A
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
Computed Descriptors for enniatin A
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1,1’-CARBONYLDIIMIDAZOLE DIETHYL AMINOMALONATE HYDROCHLORIDE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 Enniatin A CAS 2503-13-1View Details
Enniatin A CAS 2503-13-1View Details
2503-13-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8