BEAUVERICIN
Synonym(s):cyclo(D -α-Hydroxyisovaleryl-L -N-methyl-Phe)3
- CAS NO.:26048-05-5
- Empirical Formula: C45H57N3O9
- Molecular Weight: 783.95
- MDL number: MFCD30541349
- EINECS: 618-347-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-29 18:06:31
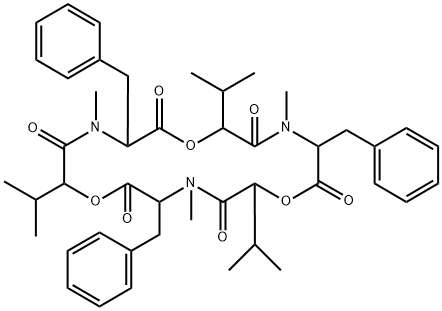
What is BEAUVERICIN?
Description
Beauvericin (CAS 26048-05-5) induces apoptosis in A549 cancer cells.1 Disrupts mitochondrial volume regulation.2 Antifungal activity acting via inhibition of both multidrug efflux and TORC1 kinase.3 Crosses the blood-brain barrier in mice.4 Inhibits HIV-1 integrase.5
Chemical properties
White powder
The Uses of BEAUVERICIN
Toxic depsipeptide with antibiotic and insecticidal effects.Beauvericin is a toxic depsipeptide with antibiotic and insecticidal effects.
The Uses of BEAUVERICIN
Beauvericin is a cyclic depsipeptide isolated from several fungal genera, notably Beauveria and Fusarium, first reported in 1969. Beauvericin exhibits broad antifungal, antibacterial, antiprotozoan and insecticidal activities. At the molecular level, beauvericin exhibits ionophoric properties, and inhibits acyl-CoA:cholesterol acyltransferase activity. Beauvericin induces apoptosis by elevating intracellular calcium levels.
What are the applications of Application
Beauvericin is an enniatin family mycotoxin
Definition
ChEBI: A trimeric cyclodepsipeptide composed from alternating methylphenylalanyl and hydroxyvaleryl residues.
References
1) Lu et al. (2016), Beauvericin-induced cell apoptosis through the mitogen-activated protein kinase pathway in human nonsmall cell lung cancer A549 cells.; J. Toxicol. Sci., 41 429 2) Tonshin et al. (2010), The Fusarium mycotoxins enniatins and beauvericin cause mitochondrial dysfunction by affecting the mitochondrial volume regulation, oxidative phosphorylation and ion homeostasis.; Toxicology, 276 49 3) Shekhar-Guturja et al. (2016), Dual action antifungal small molecule modulates multidrug efflux and TOR signaling; Nat. Chem. Biol., 12 867 4) Taevernier et al. (2016), Blood-brain barrier transport kinetics of the cyclic depsipeptide mycotoxins beauvericin and enniatins; Toxicol. Lett., 258 175 5) Shin et al. (2009), Beauvericin and enniatins H,I and MK1688 are new potent inhibitors of human immunodeficiency virus type-1 integrase; J. Antibiot. (Tokyo), 62 687
Properties of BEAUVERICIN
| Melting point: | 93-94℃ |
| Boiling point: | 975.6±65.0 °C(Predicted) |
| Density | 1.126±0.06 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | Soluble in DMSO (30 mg/ml) |
| form | neat |
| pka | -1.02±0.70(Predicted) |
| form | Solid |
| color | White |
| Stability: | Stable for 2 years from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 3 months. |
Safety information for BEAUVERICIN
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for BEAUVERICIN
| InChIKey | GYSCAQFHASJXRS-ABKJGVMTSA-N |
New Products
(R)-1-Boc-3-hydroxypyrrolidine Methyl (R)-1-Boc-4,4-difluoropyrrolidine-2-carboxylate 2,2-Difluoropropylamine hydrochloride tert-butyl 3-bromoazetidine-1-carboxylate DIFLUOROACETIC ANHYDRIDE 2,2-Difluoropropionic acid Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) Dibenzoyl Peroxide Titanium Dioxide 2-(Methylthio) Benzonitrile Sodium Acetate Anhydrous Allopurinol 1,5-DibromopentaneRelated products of tetrahydrofuran

You may like
-
 Beauvericin CAS 26048-05-5View Details
Beauvericin CAS 26048-05-5View Details
26048-05-5 -
![Cis-2-(Bromomethyl)-2-(2,4-Dichlorophenyl)-1,3-Dioxolane-4-Ylmethyl Benzoate [CBB] 61397-56-6 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) Cis-2-(Bromomethyl)-2-(2,4-Dichlorophenyl)-1,3-Dioxolane-4-Ylmethyl Benzoate [CBB] 61397-56-6 99%View Details
Cis-2-(Bromomethyl)-2-(2,4-Dichlorophenyl)-1,3-Dioxolane-4-Ylmethyl Benzoate [CBB] 61397-56-6 99%View Details
61397-56-6 -
 287930-77-2 / 142569-70-8 99%View Details
287930-77-2 / 142569-70-8 99%View Details
287930-77-2 / 142569-70-8 -
 Ethyl-2-Chloroacetoacetate 609-15-4View Details
Ethyl-2-Chloroacetoacetate 609-15-4View Details
609-15-4 -
 CIS- BROMO BENZOATEView Details
CIS- BROMO BENZOATEView Details
61397-56-6 -
 609-15-4View Details
609-15-4View Details
609-15-4 -
![1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) 1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%View Details
1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%View Details
221615-75-4 -
 27143-07-3View Details
27143-07-3View Details
27143-07-3
