Eltrombopag
- CAS NO.:496775-61-2
- Empirical Formula: C25H22N4O4
- Molecular Weight: 442.47
- MDL number: MFCD20926253
- EINECS: 610-469-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-25 11:31:46
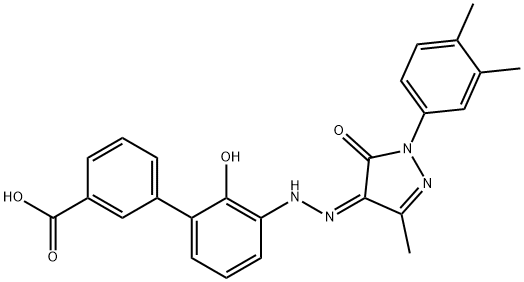
What is Eltrombopag?
Absorption
Peak absorption of Eltrombopag occurs around 2-6 hours following oral administration, and the total oral absorption of drug-related material following a 75 mg dose was estimated to be at least 52%.
Toxicity
Eltrombopag may cause hepatotoxicity, especially if administered in combination with interferon and ribavirin in patients with chronic hepatitis C (may increase the risk of hepatic decompensation).
Description
ITP is an autoimmune disease in which antiplatelet antibodies accelerate the destruction of platelets. In addition, platelet production can be
impaired because the antiplatelet antibodies can also damage megakaryocytes. Although the thrombocytopenia of ITP can be severe, signs of
bleeding are usually only minor.
Eltrombopag is an oral, small-molecule, nonpeptide TPO-receptor agonist. It initiates TPO-receptor signaling by interacting with the
transmembrane domain of the receptor, thereby inducing proliferation
and differentiation of cells in the megakaryocytic lineage. Eltrombopag is
the second TPO-receptor agonist to reach the market behind romiplostim
(Nplate), a recombinant fusion protein launched in 2008. Both drugs are
specifically indicated for the treatment of thrombocytopenia in patients
with chronic ITP who have had an insufficient response to corticosteroids,
immunoglobulins, or splenectomy. Eltrombopag is supplied as the ethanolamine salt (also known as eltrombopag olamine).
The most common adverse reactions associated with eltrombopag were nausea, vomiting, mennorrhagia, myalgia, paresthesia and cataracts. Eltrombopag is derived in five synthetic steps starting from 2-bromo-6-nitroanisole, via Suzuki coupling reaction with 3-carboxyphenylboronic acid to a biphenyl intermediate, followed by cleavage of the methyl ether moiety with hydrobromic acid, and reduction of the nitro group to an amino group under catalytic hydrogenation conditions. Subsequently, the amino group is diazotized with sodium nitrite and hydrochloric acid, and the diazonium intermediate is condensed with 1-(3,4-dimethylphenyl)-3-methyl-2,5-dihydro-1H-pyrazol-5-one to produce eltrombopag.
Chemical properties
Orange to Red Solid
Originator
Ligand Pharmaceuticals (US)
The Uses of Eltrombopag
Eltrombopag (SB-497115-GR, Promacta, Revolade) is a small molecule agonist of the c-mpl (TpoR) receptor with an IC50 of 0.69 μM for the inhibition of hERG K+ channel tail current. In preclinical studies, the compound was shown to interact selectively with
The Uses of Eltrombopag
An agonist of the Thrombopoietin (Tpo) receptor, used as treatment for thrombocytopenia.
Background
Eltrombopag is used to treat low blood platelet counts in adults with chronic immune (idiopathic) thrombocytopenia (ITP), when certain other medicines, or surgery to remove the spleen, have not worked well enough. ITP is a condition that may cause unusual bruising or bleeding due to an abnormally low number of platelets in the blood. Eltrombopag has also been recently approved (late 2012) for the treatment of thrombocytopenia (low blood platelet counts) in patients with chronic hepatitis C to allow them to initiate and maintain interferon-based therapy.
Indications
Thrombopoietin receptor agonists are pharmaceutical agents that stimulate platelet production in the bone marrow. In this, they differ from the previously discussed agents that act by attempting to curtail platelet destruction.
What are the applications of Application
Eltrombopag is an agonist of the c-Mpl (thrombopoietin receptor)
Definition
ChEBI: A hydrazine in which each nitrogen atom is substituted, one by a 3'-carboxy-2-hydroxy[1,1'-biphenyl]-3-yl group and the other by a 1-(3,4-dimethylphenyl)-3-methyl-5-oxo-1,5-dihydro-4H-pyrazol-4-ylidene group. A small molecule agonist of the c-mpl (TpoR) receptor (the physiological target of the hormone thrombopoietin), it has been developed as a medication for conditions that lead to thrombocytopenia (abnormally low platelet counts).
brand name
Promacta
Clinical Use
Thrombopoetin receptor agonist:
Treatment of chronic immune idiopathic
thrombocytopenic purpura (ITP)
Chronic hepatitis C associated thrombocytopenia
(HCV)
Severe aplastic anaemia
Synthesis
The synthesis of Eltrombopag is as follows:
At room temperature, add 8.02g (20.0mmol) compound of formula V, 3.98g (24.0mmol) 3-carboxyphenylboronic acid, 8.48g (80.0mmol) sodium carbonate, 1.46g (2.0mmol) PdCl2dppf into a 250mL three-necked flask, 80mL ethanol, 65mL water, nitrogen protection, heated to 80, reacted for 10h, cooled to room temperature, added 60mL water, filtered, the filtrate was adjusted to pH 1-2 with 2M hydrochloric acid, a red solid was precipitated, filtered, and the filter cake was ethanol/water ( v/v=1:1, 40mL) beating, filtering, and drying to obtain 7.58g, the yield is 85.8%, and the purity is 99.40%.
Drug interactions
Potentially hazardous interactions with other drugs Ciclosporin: concentration of eltrombopag reduced. Statins: increased rosuvastatin concentration, may need to reduce rosuvastatin dose.
Metabolism
Eltrombopag is predominantly through pathways including cleavage, oxidation, and conjugation with glucuronic acid, glutathione, or cysteine. In vitro studies suggest that CYP1A2 and CYP2C8 are responsible for the oxidative metabolism of eltrombopag. UGT1A1 and UGT1A3 are responsible for the glucuronidation of eltrombopag.
Metabolism
Mainly hepatically metabolised through cleavage,
oxidation by cytochrome P450 isoenzymes CYP1A2
and CYP 2C8 and conjugation with glucuronic acid,
glutathione, or cysteine.
Approximately 31% of a dose is eliminated in the
urine as metabolites, and about 59% in the faeces (20%
unchanged).
References
1) Xie?et al. (2018), Pharmacological characterization of eltrombopag, a novel orally active human thrombopoietin receptor agonist; J. Cell. Mol. Med.,?22?5367 2) Erickson-Miller?et al.?(2008),?Preclinical activity of eltrombopag (SB-497115), an oral, nonpeptide thrombopoietin receptor agonist; Stem Cells,?27?424 3) Alvarado?et al.?(2019),?Eltrombopag maintains human hematopoietic stem and progenitor cells under inflammatory conditions mediated by IFN-γ; Blood,?133?2043 4) Guenther?et al.?(2019),?Eltrombopag promotes DNA repair in human hematopoietic stem and progenitor cells; Exp. Hematol.?73?1 5) Vlachodimitropoulou?et al.?(2017),?Eltrombopag: a powerful chelator of cellular or extracellular iron (II) alone or combined with a second chelator; Blood,?130?1923 6) Kao?et al.?(2018),?Thrombopoietin receptor-independent stimulation of hematopoietic stem cells by eltrombopag; Science Transl. Med.,?10?eaas9563
Properties of Eltrombopag
| Melting point: | 242-244°C |
| Boiling point: | 656.8±65.0 °C(Predicted) |
| Density | 1.33 |
| storage temp. | Keep in dark place,Inert atmosphere,Room temperature |
| solubility | Soluble in DMSO (up to 55 mg/ml) or in ethanol (up to 14 mg/ml). |
| form | solid |
| pka | -1.26±0.40(Predicted) |
| color | Orange |
| Stability: | Stable for 2 years from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20° for up to 3 months. |
| InChI | InChI=1S/C25H22N4O4/c1-14-10-11-19(12-15(14)2)29-24(31)22(16(3)28-29)27-26-21-9-5-8-20(23(21)30)17-6-4-7-18(13-17)25(32)33/h4-13,26,30H,1-3H3,(H,32,33)/b27-22- |
Safety information for Eltrombopag
Computed Descriptors for Eltrombopag
| InChIKey | XDXWLKQMMKQXPV-QYQHSDTDSA-N |
| SMILES | C1(C2=CC=CC(N/N=C3\C(=O)N(C4=CC=C(C)C(C)=C4)N=C\3C)=C2O)=CC=CC(C(O)=O)=C1 |
Eltrombopag manufacturer
ALS India Life Sciences Pvt. Ltd
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran




You may like
-
 Eltrombopag 98%View Details
Eltrombopag 98%View Details -
 Eltrombopag 98%View Details
Eltrombopag 98%View Details -
 496775-61-2 Eltrombopag 98%View Details
496775-61-2 Eltrombopag 98%View Details
496775-61-2 -
 496775-61-2 98%View Details
496775-61-2 98%View Details
496775-61-2 -
 Eltrombopag 98% (HPLC) CAS 496775-61-2View Details
Eltrombopag 98% (HPLC) CAS 496775-61-2View Details
496775-61-2 -
 Eltrombopag CAS 496775-61-2View Details
Eltrombopag CAS 496775-61-2View Details
496775-61-2 -
 496775-61-2 Eltrombopag 99.97%View Details
496775-61-2 Eltrombopag 99.97%View Details
496775-61-2 -
 Eltrombopag API USP/BP/EP/JP/IPView Details
Eltrombopag API USP/BP/EP/JP/IPView Details
496775-61-2
