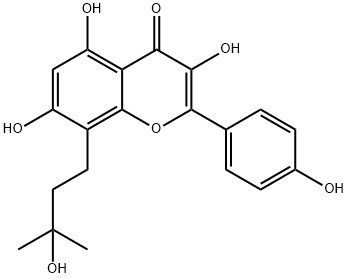
Icariin
Synonym(s):4′-O-methyl-8-γ,γ-dimethylallylkaempferol-3-rhamnoside-7-glucoside
- CAS NO.:489-32-7
- Empirical Formula: C33H40O15
- Molecular Weight: 676.66
- MDL number: MFCD00210516
- EINECS: 610-440-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
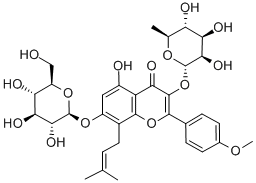
What is Icariin?
Chemical properties
Light yellow to yellow solid
The Uses of Icariin
Icariin has been used:
- in the preparation of topical treatment to determine its effects on the improvement of cutaneous wound healing in rats
- to test its analgesic effects on lower back pain (LBP) in rats
- as a potential treatment in osteoporosis condition in rats
- to study its effects on palmitate (PA)-induced insulin resistance in skeletal muscle C2C12 myotubes
- as a neuroprotective agent to study its effects on amyloid-β (Aβ)-induced neuronal insulin resistance in human neuroblastoma SK-N-MC cells
The Uses of Icariin
Icariin has been used as a test material to investigate its, in vitro effect in promoting mouse hair follicle growth, which is assessed by vibrissae hair follicle (VHF) organ-culture model. It is also used as a hepatoprotective.
What are the applications of Application
Icariin is an inhibitor of recombinant PDE5
Definition
ChEBI: Icariin is a member of the class of flavonols that is kaempferol which is substituted at position 8 by a 3-methylbut-2-en-1-yl group and in which the hydroxy groups at positions 3, 4', and 7 have been converted to the corresponding 6-deoxy-alpha-L-mannopyranoside, methyl ether, and beta-D-glucopyranoside, respectively. A phoshphodiesterase-5 inhibitor, it is obtained from several species of plants in the genus Epimedium and is thought to be the main active ingredient of the Chinese herbal medicine Herba Epimedii (yinyanghuo). It has a role as a bone density conservation agent, a phytoestrogen, an EC 3.1.4.35 (3',5'-cyclic-GMP phosphodiesterase) inhibitor and an antioxidant. It is a glycosyloxyflavone and a member of flavonols.
General Description
Icariin is a prenylated flavonol and the major bioactive compound found in Epimedium herb.
Biological Activity
icariin exhibits inhibitory effects on cgmp-specific phosphodiesterase pde5 and camp-specific phosphodiesterase pde4 activities. phosphodiesterase (pde) is a crucial regulator of camp/pka signaling. pdes are encoded by 21 genes which can be sdivided into 11 families according to the substrate specificities and subcellular localization. pdes are widely expressed in neurons. pde5 has been implicated in regulating some physiological processes such as smooth muscle relaxation and neuronal survival. pde4 has been associated with the darpp-32 signaling pathway and dopaminergic neurotransmission [1].
Biochem/physiol Actions
Icariin is a potent neuroprotective agent in neurodegenerative disorders and other disorders affecting the nervous system. It elicits anti-aging properties in unfertilized oocytes against age-related damage. Icariin exerts anti-inflammatory and antifibrotic properties aiding protection in chronic kidney disease (CKD)-associated renal fibrosis in mouse model. It also serves as an excellent antidiabetic and anti-atherosclerotic agent. Icariin is an excellent anti-cancer agent.
Mechanism of action
Icariin is known as an indicative constituent of the Epimedium genus, which has been commonly used in Chinese herbal medicine to enhance treat impotence and improve sexual function, as well as for several other indications for over 2000 years. Icariside II is a intestinal metabolite of icariin. Their inhibition profile are different. Icaritin is a potent inhibitor of UGT1A7 and UGT1A9 but icariside II is a potent inhibitor of UGT1A4, UGT1A7, UGT1A9, and UGT2B7.
in vitro
icariin inhibited the activity of pde5 and pde4 in a dose- andconcentration-dependent manner. the ic50of icariin on pde5 was 0.43 μm and the ic50 on pde4 was 73.50 μm. icariin showed a selective inhibitory effect on cgmp-specific pde5 compared to camp-specific pde4 [2].icariincould also enhance the osteogenic differentiation of rat primary bone marrow stromal cells [3].
in vivo
in castrated rats, a 4-week oral administration of icariinat 1 mg/kg/day and 5 mg/kg/day improved the erectile function and increased nnos and inos expression [4].icariin also showed its effect on stimulating angiogenesis in human endothelial cells [5].
References
nishi a, kuroiwa m, miller d b, et al. distinct roles of pde4 and pde10a in the regulation of camp/pka signaling in the striatum[j]. the journal of neuroscience, 2008, 28(42): 10460-10471.xin z c, kim e k, lin c s, et al. effects of icariin on cgmp-specific pde5 and camp-specific pde4 activities[j]. asian journal of andrology, 2003, 5(1): 15-18.chen k m, ge b f, ma h p, et al. icariin, a flavonoid from the herb epimedium enhances the osteogenic differentiation of rat primary bone marrow stromal cells[j]. die pharmazie-an international journal of pharmaceutical sciences, 2005, 60(12): 939-942.liu w j, xin z c, xin h, et al. effects of icariin on erectile function and expression of nitric oxide synthase isoforms in castrated rats[j]. asian journal of andrology, 2005, 7(4): 381-388.chung b h, kim j d, kim c k, et al. icariin stimulates angiogenesis by activating the mek/erk-and pi3k/akt/enos-dependent signal pathways in human endothelial cells[j]. biochemical and biophysical research communications, 2008, 376(2): 404-408.
Properties of Icariin
| Melting point: | 223-225 ºC |
| Boiling point: | 948.5±65.0 °C(Predicted) |
| alpha | D15 -87.09° (in pyridine) |
| Density | 1.55 |
| RTECS | DJ2980500 |
| storage temp. | 2-8°C |
| solubility | DMSO: soluble50mg/mL, clear, colorless to dark yellow |
| form | Powder |
| pka | 5.90±0.40(Predicted) |
| color | light yellow to yellow |
| λmax | 350nm(MeOH)(lit.) |
| Merck | 14,3617 |
| Stability: | Light Sensitive |
Safety information for Icariin
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for Icariin
| InChIKey | TZJALUIVHRYQQB-XLRXWWTNSA-N |
New Products
1-Boc-4-cyanopiperidine tert-Butyl carbazate 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE TETRABUTYLAMMONIUM CYANIDE TETRAHYDRO-2H-PYRAN-3-OL 3-Pyridineacrylic acid Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% Tadalafil Clopidogrel bisulfate Sitagliptin Phosphate Monohydrate Cabergoline Fexofinadine HCl Etoricoxib 4-Amino Acetophenone 2-Chloro Acetophenone Amlodipine Base 2,3,5-Triiodobenzoic Acid Pyrrolidine Diiodo PentoxideRelated products of tetrahydrofuran


You may like
-
 Icariin CAS 489-32-7View Details
Icariin CAS 489-32-7View Details
489-32-7 -
 Icariin, ≥94% CAS 489-32-7View Details
Icariin, ≥94% CAS 489-32-7View Details
489-32-7 -
 Icariin, ≥96% CAS 489-32-7View Details
Icariin, ≥96% CAS 489-32-7View Details
489-32-7 -
 Icariin CAS 489-32-7View Details
Icariin CAS 489-32-7View Details
489-32-7 -
 Icariin CAS 489-32-7View Details
Icariin CAS 489-32-7View Details
489-32-7 -
 Icariin CAS 489-32-7View Details
Icariin CAS 489-32-7View Details
489-32-7 -
 366789-02-8 Riveroxaban 98%View Details
366789-02-8 Riveroxaban 98%View Details
366789-02-8 -
 Carvedilol 98%View Details
Carvedilol 98%View Details
72956-09-3
