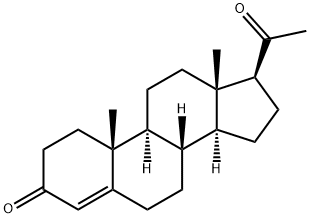
Dydrogesterone
Synonym(s):9β,10α-Pregna-4,6-diene-3,20-dione
- CAS NO.:152-62-5
- Empirical Formula: C21H28O2
- Molecular Weight: 312.45
- MDL number: MFCD00867864
- EINECS: 205-806-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-22 14:18:24

What is Dydrogesterone?
Absorption
Rapidly absorbed in the gastrointestinal tract with a bioavailability of 28%.
Toxicity
No serious or unexpected toxicity has been observed with dydrogesterone. In acute toxicity studies, the LD50 doses in rats exceeded 4,640mg/kg for the oral route.
Description
Dydrogesterone is a synthetic progestogen that has no estrogenic, androgenic, glucocorticoid, or anabolic effects, although it is an agonist of progesterone receptors (EC50 = 12.3 nM) and an antagonist of androgen, mineralocorticoid, and glucocorticoid receptors (IC50s = 28.6, 82.7, and 363 nM, respectively). Dydrogesterone protects against sound stress-induced abortion in mice when administered at a dose of 1.25 mg per animal prior to the stressor on day five of pregnancy. Formulations containing dydrogesterone have been used in hormone replacement therapy, in the treatment of menstrual disorders, and to prevent miscarriage.
Chemical properties
Light Yellow Solid
Originator
Duphaston,Duphar,UK,1961
The Uses of Dydrogesterone
A synthetic progestin largely used in hormone therapy, on the central nervous system by studying two markers of the neuroendocrine function: the neurosteroid allopregnanolone and the opioid .beta.-endorphin. Progestogen.
The Uses of Dydrogesterone
central stimulant, convulsant
Indications
Used to treat irregular duration of cycles and irregular occurrence and duration of periods caused by progesterone deficiency. Also used to prevent natural abortion in patients who have a history of habitual abortions.
Background
A synthetic progestational hormone with no androgenic or estrogenic properties. Unlike many other progestational compounds, dydrogesterone produces no increase in temperature and does not inhibit ovulation.
What are the applications of Application
Dydrogesterone is a progestin
What are the applications of Application
Dydrogesterone is a synthetic progestin. Dydrogesterone alone or in combination with estrogen to endothelial cells results in neutral effects on NO synthesis and on the activity and expression of eNOS.
Definition
ChEBI: Dydrogesterone is a 3-oxo-Delta(4) steroid and a 20-oxo steroid. It has a role as a progestin.
Manufacturing Process
A solution of 7.5 grams of retroprogesterone in 500 ml of freshly distilled
tertiary butyl alcohol was refluxed with 12.75 grams of finely powdered
chloranil, while stirring, for 5 hours in a nitrogen atmosphere. After cooling, 2
liters of water were added and extraction was performed three times with 200
ml of methylene dichloride. The combined extracts were then diluted with 1
liter of petroleum ether (40-60°C) washed successively with 100 ml of diluted
Na2SO4, four times with 75 ml of 1 N NaOH, and then water to neutral
reaction.
By drying this solution on Na2SO4, and evaporating to dryness (last part in
vacuo) 3.7 grams of crystalline residue was obtained. This residue was then
dissolved in benzene. Filtration in benzene filtered through 35 grams of
alumina (according to Brockmann was done and then the alumina was eluted
with benzene. Evaporation of the benzene yielded 3.11 grams of crystalline
residue. By crystallization with 15 ml of acetone at room temperature (at
lower temperatures a by-product crystallized out) 900 mg of crystals, with a
melting point of 165°-170°C were obtained. Transfer of the acetone mother
liquor into a mixture of ethanol and hexane yielded 1.7 grams of a solid
substance with a melting point of 130° to 145°C. This solid was then
recrystallized with acetone at room temperature, yielding 600 mg of a solid
with a melting point of 166° to 171°C. The two fairly pure fractions (600 mg
and 900 mg) yielded, after crystallization with a mixture of acetone and
hexane, finally 1.0 gram of 6-dehydroretroprogesterone, melting point 169° to
170°C. From the mother liquors an additional fraction of 0.44 gram with a
melting point of 168° to 169°C was obtained.
brand name
Gynorest (Solvay Pharmaceuticals).
Therapeutic Function
Progestin
Pharmacokinetics
Dydrogesterone is an orally active progestogen which acts directly on the uterus, producing a complete secretory endometrium in an estrogen-primed uterus. At therapeutic levels, dydrogesterone has no contraceptive effect as it does not inhibit or interfere with ovulation or the corpus luteum. Furthermore, dydrogesterone is non-androgenic, non-estrogenic, non-corticoid, non-anabolic and is not excreted as pregnanediol. Dydrogesterone helps to regulate the healthy growth and normal shedding of the uterus lining. Therefore, it may be useful in the treatment of menstrual disorders such as absent, irregular or painful menstrual periods, infertility, premenstrual syndrome and endometriosis.
Side Effects
Depression, feeling dizzy, vomiting, liver problems (jaundice (yellow eyes), weakness, stomach pain, generalized discomfort), allergic skin reactions (rashes, itching, redness), weight gain.
Synthesis
Dissolving progesterone, and carrying out photochemical reaction to obtain 9 beta, 10 alpha-pregn-4-ene-3, 20-diketone; mixing the 9 beta, 10 alpha-pregn-4-ene-3, 20-dione, an organic solvent and a benzoquinone oxidant, and carrying out dehydrogenation oxidation reaction to obtain dydrogesterone.
Metabolism
Metabolism is complete to a 20-dihydrodydrogesterone (DHD) metabolite.
Properties of Dydrogesterone
| Melting point: | 168-173°C |
| Boiling point: | 392.36°C (rough estimate) |
| alpha | D25 -484.5° (chloroform) |
| Density | 1.0697 (rough estimate) |
| refractive index | 1.4900 (estimate) |
| storage temp. | 2-8°C |
| solubility | Practically insoluble in water, soluble in acetone, sparingly soluble in ethanol (96 per cent). |
| form | neat |
| form | Solid |
| color | White to Light Yellow |
| InChI | InChI=1/C21H28O2/c1-13(22)17-6-7-18-16-5-4-14-12-15(23)8-10-20(14,2)19(16)9-11-21(17,18)3/h4-5,12,16-19H,6-11H2,1-3H3/t16-,17+,18-,19+,20+,21+/s3 |
| CAS DataBase Reference | 152-62-5(CAS DataBase Reference) |
Safety information for Dydrogesterone
Computed Descriptors for Dydrogesterone
| InChIKey | JGMOKGBVKVMRFX-HQZYFCCVSA-N |
| SMILES | C[C@@]12CCC(=O)C=C1C=C[C@@]1([H])[C@]3([H])CC[C@H](C(=O)C)[C@@]3(C)CC[C@@]21[H] |&1:1,10,12,16,20,24,r| |
Dydrogesterone manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran


You may like
-
 152-62-5 Dydrogesterone 98%View Details
152-62-5 Dydrogesterone 98%View Details
152-62-5 -
 Dydrogesterone 99%View Details
Dydrogesterone 99%View Details -
 Dydrogesterone 98%View Details
Dydrogesterone 98%View Details
152-62-5 -
 152-62-5 Dydrogesterone 98%View Details
152-62-5 Dydrogesterone 98%View Details
152-62-5 -
 Dydrogesterone 99%View Details
Dydrogesterone 99%View Details -
 Dydrogesterone 98.00% CAS 152-62-5View Details
Dydrogesterone 98.00% CAS 152-62-5View Details
152-62-5 -
 DH33 CASView Details
DH33 CASView Details -
 Dydrogesterone API IP BP USPView Details
Dydrogesterone API IP BP USPView Details
152-62-5
