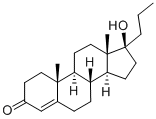
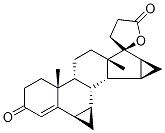
Drospirenone
Synonym(s):6β,7β:15β,16β -Dimethylene-3-oxo-17α-pregn-4-ene-21,17-carbolactone;Dihydrospirorenone;Drospirenone
- CAS NO.:67392-87-4
- Empirical Formula: C24H30O3
- Molecular Weight: 366.5
- MDL number: MFCD00867350
- EINECS: 266-679-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 20:33:22
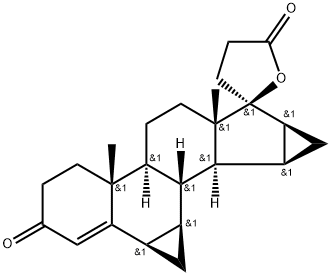
What is Drospirenone?
Absorption
The absolute bioavailability of drospirenone is approximately 76% due to first-pass effects. The maximum plasma concentration of drospirenone occurs within 1 to 2 hours after oral administration and is estimated to range between 60 and 87 ng/mL. A European prescribing monograph for the combination product of estradiol and drospirenone indicates that drospirenone is both completely and rapidly absorbed. It reports a Cmax of 21.9 ng/ml, achieved approximately 1-hour post-administration. The absolute bioavailability is reported to range between 76 to 85%.
Toxicity
The oral LD50 of drospirenone in rats is >2000 mg/kg.
Overdose information
An overdose of drospirenone, like other oral contraceptives, may lead to cause nausea or withdrawal bleeding. For drospirenone in particular, as an analog of spironolactone, may affect the levels of serum sodium and potassium. Their concentrations should be monitored in cases of overdose in addition to monitoring from metabolic acidosis and hyperkalemia, which may also result.
Description
The steroid drospirenone in combination with the estrogen agonist ethinylestradiol was introduced in Germany as a new oral contraceptive for women. This analog of the aldosterone antagonist spironoiactone can be synthesized in five steps from 3β-hydroxy- 15β,16β-methylene-5-androsten-l7-one. Since its binding profile for steroid receptors is very similar to progesterone, drospirenone mimics the progestogen agonistic activity as well as the anti-androgenic and anti-mineralocorticoid properties of the endogenous hormone. In rats, drospirenone inhibited ovulation at 6.3 mglkglday p.o. Compared with currently available progestins which lack anti-mineralocorticoid activity, drospirenone did not cause weight gain that could result from fluid retention in clinical studies. Its combination with ethinylestradiol was well tolerated and did not engender adverse effects on blood pressure or plasma lipid levels. Drospirenone is rapidly absorbed in man with an oral bioavailability of 76%. It is extensively metabolized since over 20 different metabolites were observed in the urine and in the feces, resulting for instance from hydrolysis of the lactone in the plasma or reductive conjugation of the enone to the 3-sulfate ester of 45 dihydrodrospirenone. Its elimination is bi-exponential with an initial and a terminal half-life of 2 and 25-33h respectively.
Description
Drospirenone is an unusual steroid with two annelated cyclopropane rings and 10 chiral centers. It is a component of some current oral contraceptive formulations and medications that control menopausal symptoms. Its biological properties were first reported in 1995 by R. Krattenmacher et al. in the journal?Contraception.
Description
Drospirenone is a synthetic progestogen that binds to the progesterone, mineralocorticoid, and androgen receptors with binding affinities of 20, 230, and 65% relative to R5020, aldosterone , and R1881, respectively. In vivo, drospirenone inhibits spontaneous ovulation in rats (ID50s = 0.3-1.0 mg/day) when administered orally or subcutaneously. Drospirenone (0.5 mg/animal) administered six times per day maintains pregnancy in ovariectomized pregnant rats. It reduces serum testosterone (Item Nos. 15645 | ISO60154) and luteinizing hormone in cynomolgus monkeys in a dose-dependent manner. Drospirenone (10 mg/animal per day) also inhibits testosterone-induced growth of the seminal vesicles and prostate in castrated rats. Formulations containing drospirenone have been used as oral contraceptives.
Chemical properties
Off-White Crystalline Powder
Originator
Schering AG (Germany)
The Uses of Drospirenone
Drospirenone has been used as a progestogen agent in pond snail and fish.
The Uses of Drospirenone
Synthetic progestogen exhibiting antimineralocorticoid and antiandrogenic activity.
The Uses of Drospirenone
antiviral
The Uses of Drospirenone
anti-cancer therapeutic
Background
Drospirenone is a synthetic progestin commonly found in the popular oral contraceptive, Yaz in combination with Ethinyl estradiol. Most recently, it was approved by both Health Canada and the FDA in combination with Estetrol as an oral contraceptive therapy. Aside from its contraceptive effects, drospirenone is used with estrogens to control acne and premenstrual dysphoric disorder (PMDD).
Drospirenone has been the subject of widespread safety concern due to the possibility of an increased risk of venous thromboembolism associated with its use. In 2012, however, a safety statement by the FDA concluded that the increase in the risk of thromboembolism resulting from the use of drospirenone remains unclear, as studies regarding this risk are conflicting. Some studies have demonstrated a significantly increased risk and some demonstrating no risk of thromboembolic events. In its statement, the FDA has mentioned that increased risk of venous thromboembolism with oral contraceptives such as drospirenone exists but remains lower than the risk of this condition during pregnancy and during the postpartum period, and this should be considered when assessing potential risks of hormonal contraceptive use.
Indications
Drospirenone, in combination with ethinyl estradiol or estetrol, is indicated as an oral contraceptive for the prevention of pregnancy. In addition to its use for contraceptive effects, this combination is used to treat moderate acne vulgaris and the symptoms of premenstrual dysphoric disorder. The drug has approved indications for combination with estrogens for the treatment of menopause-associated symptoms, such as vasomotor symptoms and vulvovaginal atrophy. Drospirenone combined with estrogen may also may aid in the prevention of osteoporosis in women who have been post-menopausal for at least a year and are not candidates for other therapies. It can sometimes be found in preparations containing estrogen and folic acid for folic acid replenishment during oral contraception.
When used for the treatment of acne vulgaris, drospirenone-containing contraceptives should only be used in women ≥14 years of age who have experienced menarche, desire oral contraception, and do not have any contraindications to oral contraceptives. Off-label uses for this drug include the treatment of menstrual irregularities, dysmenorrhea, hirsutism, and endometriosis.
What are the applications of Application
Drospirenone is a synthetic progestogen with antiandrogenic activity
Definition
ChEBI: Drospirenone is a steroid lactone and a 3-oxo-Delta(4) steroid. It has a role as a contraceptive drug, an aldosterone antagonist and a progestin.
Manufacturing Process
2.75 g of trimethyl sulfoxonium iodide is stirred in 57 ml of dimethyl sulfoxide with 341 mg of 80% sodium hydride oil suspension for 2 h at room temperature. The almost clear solution is combined under nitrogen with 2.0 g of 15α,16α-methylene-3-oxo-4,6-androstadiene-[17(β-1')-spiro- 5']perhydrofuran-2'-one and agitated for 24 h at room temperature. The mixture is then stirred into ice water, the thus-obtained precipitate is filtered off, washed with water, and taken up in methylene chloride. After drying and evaporation, the residue is purified by repeated preparative layer chromatography, thus obtaining 520 mg of 6β,7β,15α,16α-dimethylene-3-oxo- 4-androstene-[17(β-1')-spiro-5']perhydrofuran-2'-one (drospirenone).
brand name
Yasmin
Therapeutic Function
Aldosterone antagonist
General Description
Drospirenone, 3-oxo-6β,7β:15β,16β-dimethylene-17α-pregn-4-en-21,17-carbolactone,differs structurally from all the other commercially availableprogestins. Its structure is similar to that of spironolactone,an MR antagonist, and it does have antimineralocorticoidactivity as well as progestational activity. It isalso reported to have some antiandrogenic effects. Thespirolactone at C17 and the two cyclopropyl groups at C6-C7 and C15-C16 contribute to these unique actions.Drospirenone is the progestin component in the newer oralcontraceptives, Yasmin and Yaz, and in the HRT product,Angeliq.
Biochem/physiol Actions
Drospirenone is a fourth-generation progestin that has antimineralocorticoid, and antiandrogenic activity in addition to potent progestogenic activity. In two recent studies drospirenone appeared to double the risk of venous thromboembolism compared to levonorgestrel, although other studies found little added risk.
Pharmacokinetics
Drospirenone inhibits the maturation of follicles and inhibits ovulation, preventing pregnancy. It has antiandrogen effects, improving acne and hirsutism. When combined with ethinyl estradiol, it has been shown to have favorable effects on the plasma lipid profile. Due to its similarity to naturally occurring progesterone, drospirenone is thought to be associated with a lower incidence of progesterone contraceptive related adverse effects, such as breast tenderness and mood swings.
A note on venous thromboembolism risk and antimineralcorticoid effects
As with other oral contraceptives, the risk of venous thromboembolism and cardiovascular events may be increased when drospirenone is taken. The risk is especially higher in smokers and women aged 35 and older. Women taking this drug should be advised not to smoke. In addition, drospirenone, due to its antimineralcorticoid effects, may increase the risk of hyperkalemia. Patients at high risk for hyperkalemia should not be administered this drug. Consult the official prescribing information for detailed and updated information on the cardiovascular and other risks associated with drospirenone use.
Metabolism
Drospirenone is heavily metabolized. The two major inactive metabolites identified are the acid form of drospirenone produced by the opening of its lactone ring, known as M11, and the 4,5-dihydro-drospirenone-3-sulfate (M14). Drospirenone also undergoes oxidative metabolism via the hepatic cytochrome enzyme CYP3A4.
Properties of Drospirenone
| Melting point: | 196-200°C |
| Boiling point: | 552.2±50.0 °C(Predicted) |
| alpha | -180 º (c=0.5, chloroform) |
| Density | 1.26±0.1 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | DMSO: ≥15 |
| form | powder |
| color | white to tan |
| optical activity | [α]/D -180 to -195°, c = 1 in methanol |
Safety information for Drospirenone
| Signal word | Danger |
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H360:Reproductive toxicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P280:Wear protective gloves/protective clothing/eye protection/face protection. P308+P313:IF exposed or concerned: Get medical advice/attention. P405:Store locked up. P501:Dispose of contents/container to..… |
Computed Descriptors for Drospirenone
| InChIKey | METQSPRSQINEEU-URUUTGNFNA-N |
| SMILES | C[C@]12CC[C@]3([H])[C@]4(CCC(=O)C=C4[C@@H]4C[C@@H]4[C@@]3([H])[C@]1([H])[C@@H]1C[C@@H]1[C@]12CCC(=O)O1)C |&1:1,4,6,13,15,16,18,20,22,23,r| |
Drospirenone manufacturer
Ralington Pharma
BDR Pharmaceuticals International Pvt Ltd
Symbiotec Pharma Lab Pvt Ltd
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 67392-87-4 Drospirenone 99%View Details
67392-87-4 Drospirenone 99%View Details
67392-87-4 -
 Drospirenone 99%View Details
Drospirenone 99%View Details
67392-87-4 -
 Drospirenone 99%View Details
Drospirenone 99%View Details -
 67392-87-4 Drospirenone 98%View Details
67392-87-4 Drospirenone 98%View Details
67392-87-4 -
 Drospirenone 67392-87-4 99%View Details
Drospirenone 67392-87-4 99%View Details
67392-87-4 -
 67392-87-4 98%View Details
67392-87-4 98%View Details
67392-87-4 -
 Drospirenone CAS 67392-87-4View Details
Drospirenone CAS 67392-87-4View Details
67392-87-4 -
 Drospirenone CAS 67392-87-4View Details
Drospirenone CAS 67392-87-4View Details
67392-87-4