Prazosin hydrochloride
Synonym(s):1-(4-Amino-6,7-dimethoxy-2-quinazolinyl)-4-(2-furanylcarbonyl)piperazine hydrochloride;Furazosin hydrochloride;Prazosin HCl;Prazosin hydrochloride
- CAS NO.:19237-84-4
- Empirical Formula: C19H22ClN5O4
- Molecular Weight: 419.86
- MDL number: MFCD00058177
- EINECS: 242-903-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-16 16:15:04
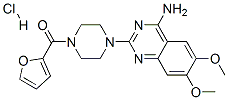
What is Prazosin hydrochloride ?
Chemical properties
Off-White to Yellow Powder
Originator
Hypovase, Pfizer, UK,1974
The Uses of Prazosin hydrochloride
Prazosin Hydrochloride (Terazosin EP Impurity K) is an antihypertensive. α1-Adrenergic blocker.
The Uses of Prazosin hydrochloride
Prazosin hydrochloride has been used:
- to block the α1adrenergic receptors that mediate sympathetic vasoconstriction in mice
- as an α1-adrenoceptor blocker,administered intragastrically in rats
- as a vasodilator,administered together with inuslin into the left ventricle of mice for the assessment of its effects on renal functions
What are the applications of Application
Prazosin hydrochloride is an andrenergic α1, α2B-adrenoceptor, & MT3 receptor antagonist
Manufacturing Process
Preparation of 2-Chloro-4-Amino-6,7-Dimethoxyquinazoline: To 800 ml of a solution of anhydrous ammonia in tetrahydrofuran at room temperature is added 30 g of 2,4-dichloro-6,7-dimethoxyquinazoline [F.H.S. Curd et al., J. Chem. Soc., p 1759 (1948)]. The mixture is stirred for 44 hours. The precipitate (29 g, MP 267° to 268°C) is filtered and recrystallized from methanol to yield 19 g of 2-chloro-4-amino-6,7-dimethoxyquinazoline, MP 302°C (dec.).
Preparation of 2-(1-Piperazinyl)-4-Amino-6,7-Dimethoxyquinazoline: To 5 g of 2-chloro-4-amino-6,7-dimethoxyquinazoline, is added 20 g of a 25% solution of piperazine in ethanol. The mixture is heated at 160°C for 16 hours in a pressure bottle. The solvent is then evaporated and the residue is recrystallized from methanol/water.
Preparation of 2[4-(2-Furoyl)-Piperazinyl]-4-Amino-6,7-Dimethoxyquinazoline: To 0.10 mol 2-(1-piperazinyl)-4-amino-6,7-dimethoxyquinazoline in 300 ml methanol is added with vigorous stirring, 0.10 mol 2-furoyl chloride. After addition is complete, the mixture is stirred for 3 hours at room temperature. The solids are filtered to give the desired product, MP 278° to 280°C.
brand name
Minipress (Pfizer).
Therapeutic Function
Antihypertensive
General Description
The antihypertensive effectsof prazosin hydrochloride, 1-(4-amino-6,7-dimethoxy-2-quinazolinyl)-4-(2-furoyl)piperazine monohydrochloride(Minipress), are caused by peripheral vasodilation as a resultof its blockade of 1-adrenergic receptors. In ligand-bindingstudies, prazosin hydrochloride has 5,000-fold greater affinityfor α1-receptors than for some α2-adrenergic receptors.
Biological Activity
α 1 and α 2B -adrenoceptor antagonist. Also a potent antagonist at the melatonin MT 3 receptor (K i = 10.2 nM). Also available as part of the α 1 -Adrenoceptor Tocriset™ and Mixed Adrenergic Tocriset™ .
Biochem/physiol Actions
Peripheral α1-adrenoceptor antagonist; vasodilator. Prazosin can be used to treat chronic posttraumatic stress disorder (PTSD). It plays a role in reducing blood pressure by relaxing blood vessels, hence used in the treatment of high blood pressure.
Clinical Use
Prazosin hydrochloride is readily absorbed, and plasmaconcentrations reach a peak about 3 hours after administration.Plasma half-life is between 2 and 3 hours. Prazosin hydrochlorideis highly bound to plasma protein; it does notcause adverse reactions, however, with drugs that might bedisplaced from their protein-binding sites (e.g., cardiac glycosides).It may cause severe orthostatic hypertension becauseof its -adrenergic blocking action, which preventsthe reflex venous constriction that is activated when an individualsits up from a prone position.
Storage
Room temperature
Purification Methods
The salt is recrystallised by dissolving it in hot MeOH, adding a small volume of MeOH/HCl (dry MeOH saturated with dry HCl gas) followed by dry Et2O until crystallisation is complete. Dry it in vacuo over solid KOH till the odour of HCl is absent. It has been recrystallised from hot H2O, the crystals are washed with H2O, and the H2O is removed azeotropically with CH2Cl2, and dried in a vacuum. [NMR and IR: Honkanen et al. J Heterocycl Chem 17 797 1980, cf Armarego & Reece Aust J Chem 34 1561 1981.] It is an antihypertensive drug and is an 1-adrenergic antagonist [Brosman et al. Proc Natl Acad Sci USA 82 5915 1985].
Properties of Prazosin hydrochloride
| Melting point: | 277 - 280 °C |
| storage temp. | 2-8°C |
| solubility | H2O: 1 mg/mL, clear, colorless |
| form | solid |
| color | white |
| Water Solubility | Soluble in dimethyl sulfoxide, ethanol, water, methanol. |
| Merck | 14,7717 |
| BRN | 4303561 |
| CAS DataBase Reference | 19237-84-4(CAS DataBase Reference) |
Safety information for Prazosin hydrochloride
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H361:Reproductive toxicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Prazosin hydrochloride
| InChIKey | WFXFYZULCQKPIP-UHFFFAOYSA-N |
Prazosin hydrochloride manufacturer
Aruvi Labs
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
![2,2'-(1,4-Piperazinediyl)bis[6,7-diMethoxy-4-quinazolinaMine]](https://img.chemicalbook.in/CAS/GIF/102839-00-9.gif)
![1-[4-(4-AMino-6,7-diMethoxy-2-quinazolinyl)-1-piperazinyl]-5-hydroxy-1-pentanone](https://img.chemicalbook.in/CAS/GIF/109678-71-9.gif)
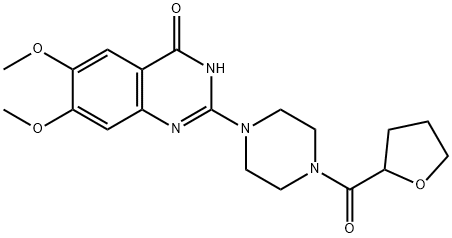
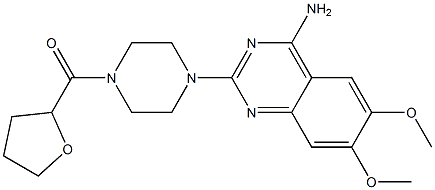
Methanone](https://img.chemicalbook.in/CAS/GIF/105356-90-9.gif)
You may like
-
 Prazosin hydrochloride 99%View Details
Prazosin hydrochloride 99%View Details -
 Prazosin hydrochloride 98%View Details
Prazosin hydrochloride 98%View Details -
 19237-84-4 98%View Details
19237-84-4 98%View Details
19237-84-4 -
 Prazosin HCl 95% CAS 19237-84-4View Details
Prazosin HCl 95% CAS 19237-84-4View Details
19237-84-4 -
 Prazosin Hydrochloride CAS 19237-84-4View Details
Prazosin Hydrochloride CAS 19237-84-4View Details
19237-84-4 -
 Prazosin hydrochloride CAS 19237-84-4View Details
Prazosin hydrochloride CAS 19237-84-4View Details
19237-84-4 -
 Prazosin hydrochloride CAS 19237-84-4View Details
Prazosin hydrochloride CAS 19237-84-4View Details
19237-84-4 -
 Prazosin hydrochloride CAS 19237-84-4View Details
Prazosin hydrochloride CAS 19237-84-4View Details
19237-84-4
