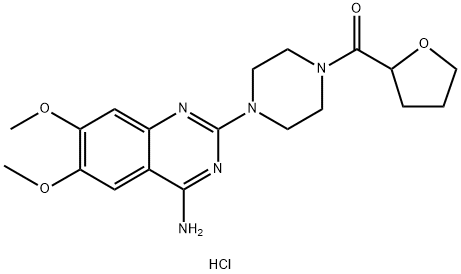
Terazosin
- CAS NO.:63590-64-7
- Empirical Formula: C19H25N5O4
- Molecular Weight: 387.43
- MDL number: MFCD00072144
- EINECS: 613-265-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-10-28 23:16:16
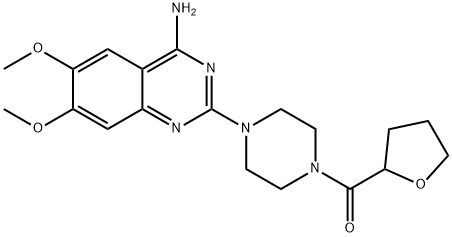
What is Terazosin?
Absorption
Approximately 90%.
Toxicity
In the event of an overdose, patients may experience hypotension. Blood pressure and heart rate should be controlled by having the patient lie down or by treating with volume expanders or if necessary vasopressors. Patients should be monitored for renal function. Because terazosin is highly protein bound, dialysis is unlikely to provide benefit to overdosing patients.
The oral LD50 in mice is 5500mg/kg.
Chemical properties
White to Off-White Crystalline Powder
The Uses of Terazosin
Terazosin is used for the same indications as is prazosin; however, it has the advantage of being able to be used once a day.
The Uses of Terazosin
An a-1-adrenergicblocker related to prazosin
The Uses of Terazosin
An α-1-adrenergicblocker related to prazosin.
Background
Terazosin is a quinazoline derivative alpha-1-selective adrenergic blocking agent indicated for benign prostatic hyperplasia and hypertension. Terazosin blocks adrenaline's action on alpha-1 adrenergic receptors, causing relaxation of smooth muscle in blood vessels and the prostate.
Indications
Terazosin is indicated for use in treating symptomatic benign prostatic hyperplasia and hypertension.
Definition
ChEBI: Terazosin is a member of quinazolines, a member of piperazines, a member of furans and a primary amino compound. It has a role as an antineoplastic agent, an antihypertensive agent and an alpha-adrenergic antagonist.
Pharmacokinetics
Terazosin is a quinazoline derivative alpha-1-selective adrenergic blocker.
Clinical Use
Alpha-adrenoceptor blocker:
Hypertension
Benign prostatic hyperplasia (BPH)
Synthesis
Terazosin, 1-(4-amino-6,7-dimethoxy-2-quinazolinyl)-4-(2-tetrahydrofuroyl)- piperazine (12.2.13), only differs from prazosin in that the furyl radical is replaced with a tetrahydrofuryl radical. It is synthesized in exactly the same manner except using 1-(2-tetrahydrofuroyl)piperazine instead of 1-(2-furoyl)piperazine [48–51].
Drug interactions
Potentially hazardous interactions with other drugs
Anaesthetics: enhanced hypotensive effect.
Antidepressants: enhanced hypotensive effect with
MAOIs.
Avanafil, vardenafil, sildenafil and tadalafil: enhanced
hypotensive effect - avoid concomitant use.
Beta-blockers: enhanced hypotensive effect;
increased risk of first dose hypotensive effect.
Calcium-channel blockers: enhanced hypotensive
effect; increased risk of first dose hypotensive effect.
Diuretics: enhanced hypotensive effect; increased
risk of first dose hypotensive effect.
Moxisylyte: possibly severe postural hypotension
when used in combination.
Metabolism
The majority of terazosin is hepatically metabolized. The metabolites recovered include 6-O-demethyl terazosin, 7-O-methyl terazosin, a piperozine derivative, and a diamine derivative.
Metabolism
Terazosin is metabolised in the liver; one of the
metabolites has antihypertensive activity.
Terazosin is excreted in faeces via the bile, and in the
urine, as unchanged drug and metabolites.
Properties of Terazosin
| Melting point: | 281-283°C |
| Boiling point: | 664.5±65.0 °C(Predicted) |
| Density | 1.332±0.06 g/cm3(Predicted) |
| storage temp. | -20°C Freezer |
| solubility | H2O: 25 mg/mL |
| pka | pKa (0.1N NaOH): 7.1(at 25℃) |
| form | powder |
| color | white to off-white |
| Water Solubility | 30.6mg/L(22.5 ºC) |
| CAS DataBase Reference | 63590-64-7(CAS DataBase Reference) |
Safety information for Terazosin
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for Terazosin
| InChIKey | VCKUSRYTPJJLNI-UHFFFAOYSA-N |
Terazosin manufacturer
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-(2,4-Dimethoxybenzyl)dihydropyrimidine-2,4(1H,3H)-dione 7-Bromo-1H-indazole N-octanoyl benzotriazole 3,4-Dibenzyloxybenzaldehyde 4-Hydrazinobenzoic acid Electrolytic Iron Powder Fmoc-Val-Cit-PAB 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 4-HYDROXY BENZYL ALCOHOL 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) S-2-CHLORO PROPIONIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 1-(4-Methylphenylsulfonyl)-1H-1,2,3-benzotriazole 1-(2-Chlorobenzyl)-4-nitro-1H-pyrazole 1-(2-Nitrophenyl)-4-phenylpiperazineRelated products of tetrahydrofuran




You may like
-
 63590-64-7 Terazosin 98%View Details
63590-64-7 Terazosin 98%View Details
63590-64-7 -
 55441-95-7 2 2-BIS(2-HYDROXYETHOXY)-1 1-BINAPHTHYL 99%View Details
55441-95-7 2 2-BIS(2-HYDROXYETHOXY)-1 1-BINAPHTHYL 99%View Details
55441-95-7 -
 Ste-Glu-AEEA-AEEA-OSUView Details
Ste-Glu-AEEA-AEEA-OSUView Details
1169630-40-3 -
 1446013-08-6 Fmoc-His-Aib-OH TFA 98%View Details
1446013-08-6 Fmoc-His-Aib-OH TFA 98%View Details
1446013-08-6 -
 127464-43-1 99%View Details
127464-43-1 99%View Details
127464-43-1 -
 Chloro Uracil 99%View Details
Chloro Uracil 99%View Details
1820-81-1 -
 2-ETHYLPYRIDINE 100-71-0 99%View Details
2-ETHYLPYRIDINE 100-71-0 99%View Details
100-71-0 -
 13162-05-5 99%View Details
13162-05-5 99%View Details
13162-05-5