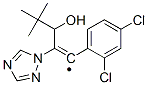
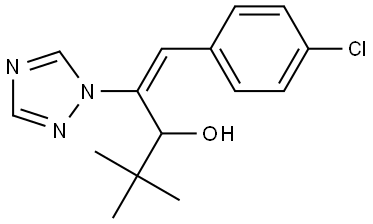
Diniconazole
Synonym(s):α-tert-Butyl-β-(2,4-dichlorophenylmethylene)-1H-1,2,4-triazole-1-ethanol;Diclopentezol
- CAS NO.:83657-24-3
- Empirical Formula: C15H17Cl2N3O
- Molecular Weight: 326.22
- MDL number: MFCD01678675
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:52
What is Diniconazole?
The Uses of Diniconazole
Diniconazole is used to control leaf and ear diseases in cereals, powdery mildew in vines, rust and black spot in roses, leaf spot in peanuts, Sigatoka disease in bananas, and Uredinales in coffee. It is also used on fruit, vegetables and ornamentals.
Definition
ChEBI: (1E)-1-(2,4-dichlorophenyl)-4,4-dimethyl-2-(1,2,4-triazol-1-yl)pent-1-en-3-ol is a member of the class of triazoles that is 4,4-dimethyl-2-(1,2,4-triazol-1-yl)pent-1-en-3-ol substituted at position 1 by a 2,4-dichlorophenyl group. It is a dichlorobenzene, an olefinic compound, a secondary alcohol and a member of triazoles.
Metabolic pathway
Diniconazole is a mixture of four isomers, which are derived from a chiral carbon atom and a double bond in the molecule. Diniconazole M [( E)-( S)-1-(2 ,4-dichlorophenyl)-4,4-dimethyl-2-1(H -1,2,4-triazol-l-yl)pent- 1-en-3-ol] is more potent than diniconazole. The major metabolic pathways of diniconazole involve oxidative attack on the pentenol side chain to give products which may form conjugates. In mammals, sulfurcontaining metabolites may be formed.
Degradation
Diniconazole is stable to heat, light and moisture. It undergoes photolysis
and irradiation of a solution in methanol with light from a high-pressure
mercury lamp for 8 hours gave three major and seven minor products, 2-
11 (Scheme 1). The major photoproduct was identified as the 2-isomer (2).
The other major products (5 and 6) were formed by photooxidation of the
CHOH group to a carbonyl group.
When diniconazole was irradiated as a thin film under ultraviolet light
(254 nm), the major product was identified as the Z-isomer. On the surface
of a sandy loam soil, under the same irradiation conditions rapid degradation
occurred with the formation of 2, 5 and 6. A thin film of diniconazole
applied to a glass plate degraded very slowly in the dark. When the
plate was placed in the sunhght, 70% of the applied material disappeared
after 5 days exposure and isomerisation occurred to give the Z-isomer
(65%). The DT50 in sunlight was 2.5 days. Under ultraviolet light (254 mn),
the Z-isomer was formed after 15 minutes irradiation, and after 5 hours
90% of the material had isomerised (Dureja and Walia, 1992).
Properties of Diniconazole
| Melting point: | 134~156℃ |
| Boiling point: | 501.1±60.0 °C(Predicted) |
| Density | 1.32 |
| vapor pressure | 2.93 x l0-3 Pa (20 °C) |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | DMSO:100.0(Max Conc. mg/mL);306.54(Max Conc. mM) Water:0.67(Max Conc. mg/mL);2.05(Max Conc. mM) |
| form | A solid |
| Water Solubility | 4 mg l-1 (25 °C) |
| pka | 12.89±0.20(Predicted) |
| color | White to off-white |
| CAS DataBase Reference | 83657-24-3(CAS DataBase Reference) |
| EPA Substance Registry System | 1H-1,2,4-Triazole-1-ethanol, .beta.-[(2,4-dichlorophenyl)methylene]-.alpha.-(1,1-dimethylethyl)-, (.beta.E)- (83657-24-3) |
Safety information for Diniconazole
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P273:Avoid release to the environment. P391:Collect spillage. Hazardous to the aquatic environment P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P501:Dispose of contents/container to..… |
Computed Descriptors for Diniconazole
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde Electrolytic Iron Powder 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 4-HYDROXY BENZYL ALCOHOL 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) S-2-CHLORO PROPIONIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 3-(Hydroxymethyl)benzoate N-Boc-2-chloroethylamine 1-Bromo-2-methoxy-3-nitrobenzene N-Methyl-3-cyclopenten-1-amine 2-Bromo-3-hydroxybenzaldehyde 1H-indazole-5-carboxamideRelated products of tetrahydrofuran



You may like
-
 Diniconazole 99% (HPLC) CAS 83657-24-3View Details
Diniconazole 99% (HPLC) CAS 83657-24-3View Details
83657-24-3 -
 Diniconazole CAS 83657-24-3View Details
Diniconazole CAS 83657-24-3View Details
83657-24-3 -
 Diniconazole CAS 83657-24-3View Details
Diniconazole CAS 83657-24-3View Details
83657-24-3 -
 7441-43-2 98%View Details
7441-43-2 98%View Details
7441-43-2 -
 1260741-78-3 6-Bromo-3-iodo-1-methyl-1H-indazole 98%View Details
1260741-78-3 6-Bromo-3-iodo-1-methyl-1H-indazole 98%View Details
1260741-78-3 -
 4-bromo-3,5-dimethylbenzenesulfonyl chloride 1581266-79-6 98%View Details
4-bromo-3,5-dimethylbenzenesulfonyl chloride 1581266-79-6 98%View Details
1581266-79-6 -
 2490430-37-8 98%View Details
2490430-37-8 98%View Details
2490430-37-8 -
 N-(5-Amino-2-methylphenyl)acetamide 5434-30-0 98%View Details
N-(5-Amino-2-methylphenyl)acetamide 5434-30-0 98%View Details
5434-30-0