Lauroyl peroxide
Synonym(s):Di(dodecanoyl) peroxide;DiDodecanoyl Peroxide;Dilauroyl peroxide;Dodecanoyl peroxide;Lauroyl peroxide
- CAS NO.:105-74-8
- Empirical Formula: C24H46O4
- Molecular Weight: 398.62
- MDL number: MFCD00008964
- EINECS: 203-326-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
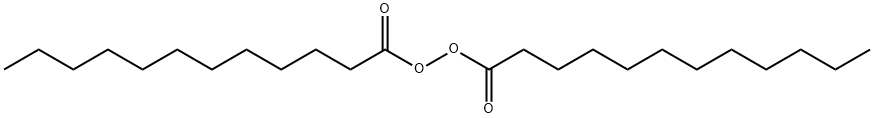
What is Lauroyl peroxide?
Chemical properties
Dilauroyl peroxide (Lauroyl peroxide) is a tasteless, coarse, white powder. Dilauroyl peroxide is not a deflagration hazard. However, it has been judged an intermediate fire hazard by Noller et al. When all the physical tests available for this peroxide are evaluated collectively, it actually ranks as a low physical hazard .
The Uses of Lauroyl peroxide
Dilauroyl peroxide is produced by reaction of lauroyl chloride with sodium peroxide. Its major use is as an initiator for vinyl chloride. It is used as a polymerization agent in the plastics industry and as a curing agent for rubber. It has also been used as a burnout agent for acetate yarns. The pharmaceutical industry uses it in topical creams in combination with antibiotics for acne treatment .
The Uses of Lauroyl peroxide
Lauroyl peroxide is used as an initiatorfor free-radical polymerization in makingpolyvinyl chloride. Lauroyl peroxide constitutesabout 4% of all organic peroxides consumptionin the United States.
The Uses of Lauroyl peroxide
Lauroyl peroxide acts as a bleaching agent, drying agent for fats, oils and waxes. Further, it serves as a polymerization initiator as well as vulcanizing agent. In addition to this, it plays an important role for high-pressure polyethylene and food used in bleaching agent.
Preparation
Dilauroyl peroxide is the symmetrical peroxide of lauric acid. It is produced by treating lauroyl chloride with hydrogen peroxide in the presence of base:
2C11H23COCl + H2O2+ 2NaOH → (C11H23CO2)2 + 2HCl
Definition
ChEBI: Lauroyl peroxide is a dicarboxylic acid.
General Description
Dilauroyl peroxide appears as a white solid with a faint soapy odor. Less dense than water and insoluble in water. Hence floats on water. Melting point 49 °C. Toxic by ingestion and inhalation. Strong skin irritant. Used as bleaching agent, drying agent for fats, oils and waxes, and as a polymerization catalyst.
Air & Water Reactions
Highly flammable. Insoluble in water.
Reactivity Profile
Dilauroyl peroxide is an oxidizing agent. Can ignite organic materials; hence a dangerous fire and explosion risk [Hawley]. Strongly reduced material such as sulfides, nitrides, and hydrides may react explosively. Vigorous reactions with other reducing agents. With charcoal sometimes ignites. [Bretherick, 5th ed., 1995, p. 1194].
Health Hazard
Contact with liquid irritates eyes and skin. Ingestion causes irritation of mouth and stomach.
Health Hazard
Lauroyl peroxide is a mild eye irritant; theirritation from 500 mg/day in rabbits’ eyeswas mild. It is nontoxic. Prolonged exposureto laboratory animals caused tumors at thesite of application. However, the evidence ofcarcinogenicity in animals is inadequate todate.
Fire Hazard
Behavior in Fire: Can increase the severity of a fire. Becomes sensitive to shock when hot. Containers may explode in a fire. May ignite or explode spontaneously if mixed with flammable materials.
Flammability and Explosibility
Not classified
Carcinogenicity
The International Agency for Research on Cancer has evaluated the carcinogenicity of lauroyl peroxide. They classified it as a Group 3 material, which means there is limited or inadequate evidence for animals and inadequate or absent information for humans . The carcinogenicity of this peroxide has primarily been studied using skin applications. After a single topical application of 10, 20, or 40 mg of lauroyl peroxide, the epidermal thickness increased markedly. This hyperplasia was characterized by a sustained production of dark basal keratinocytes. This peroxide is inactive as a tumor initiator or as a complete carcinogen. However, it is as effective as benzoyl peroxide as a skin tumor promoter.
storage
Store in a well-ventilated, cool area, isolatedfrom other chemicals. It is shippedin fiber drums not exceeding 100 lb. Smallamounts may be shipped in 1-lb fiberboardboxes.
Purification Methods
Crystallise it from n-hexane or *benzene and store it below 0o. Potentially EXPLOSIVE. [cf Beilstein 2 IV 1102.]
Waste Disposal
Although lauroyl peroxide is relatively lesshazardous, it is recommended that to handlespills and disposal, the same safety measuresbe followed as those for other hazardousorganic peroxides.
Properties of Lauroyl peroxide
| Melting point: | 53-57 °C(lit.) |
| Boiling point: | 441.76°C (rough estimate) |
| Density | 0,91 g/cm3 |
| vapor density | 13.7 (vs air) |
| vapor pressure | 0Pa at 20℃ |
| refractive index | 1.4460 (estimate) |
| storage temp. | 2-8°C |
| solubility | Chloroform (Slightly), DMSO (Slightly, Sonicated) |
| form | Powder |
| color | White |
| Odor | wh. coarse powd., faint odor, tasteless |
| Water Solubility | Soluble in oils and organic solvents. Slightly soluble in alcohol. Insoluble in water. |
| BRN | 1804936 |
| CAS DataBase Reference | 105-74-8(CAS DataBase Reference) |
| IARC | 3 (Vol. 36, Sup 7, 71) 1999 |
| EPA Substance Registry System | Peroxide, bis(1-oxododecyl) (105-74-8) |
Safety information for Lauroyl peroxide
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02 |
| GHS Hazard Statements |
H242:Self-reactive substances and mixtures; and Organic peroxides |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P234:Keep only in original container. P235:Keep cool. P280:Wear protective gloves/protective clothing/eye protection/face protection. P370+P378:In case of fire: Use … for extinction. P410:Protect from sunlight. |
Computed Descriptors for Lauroyl peroxide
| InChIKey | YIVJZNGAASQVEM-UHFFFAOYSA-N |
Lauroyl peroxide manufacturer
Veekay Chemicals
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 105-74-8 Dilauroyl peroxide 98%View Details
105-74-8 Dilauroyl peroxide 98%View Details
105-74-8 -
 Lauroyl peroxide CAS 105-74-8View Details
Lauroyl peroxide CAS 105-74-8View Details
105-74-8 -
 Di-Lauroyl Peroxide CAS 105-74-8View Details
Di-Lauroyl Peroxide CAS 105-74-8View Details
105-74-8 -
 Lauroyl peroxide CAS 105-74-8View Details
Lauroyl peroxide CAS 105-74-8View Details
105-74-8 -
 Luperox® LP, Lauroyl peroxide CAS 105-74-8View Details
Luperox® LP, Lauroyl peroxide CAS 105-74-8View Details
105-74-8 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1