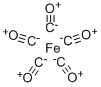DIIRON NONACARBONYL
Synonym(s):Enneacarbonyldiiron;Iron enneacarbonyl;Nonacarbonyldiiron
- CAS NO.:15321-51-4
- Empirical Formula: C9Fe2O9
- Molecular Weight: 363.78
- MDL number: MFCD00151465
- EINECS: 239-359-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:52
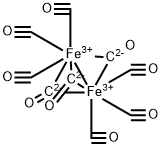
What is DIIRON NONACARBONYL?
Description
This carbonyl is obtained as dark yellow platelets by exposing solutions of the pentacarbonyl in organic solvents to sunlight or ultraviolet irradiation.
Chemical properties
yellow to orange platelets
The Uses of DIIRON NONACARBONYL
It is an important reagent in organometallic chemistry and of occasional use in organic synthesis.
The Uses of DIIRON NONACARBONYL
Reacts with phenylsilanes to give triply bridged diiron complexes.
Purification Methods
Wash it with EtOH and Et2O, then dry it in air. It sublimes at 35o at high vacuum. It forms dark yellow plates which are stable for several days when kept in small amounts. Large amounts, especially when placed in a desiccator, spontaneously ignite in a period of one day. It decomposes in moist air. It is insoluble in hydrocarbon solvents but forms complexes with several organic compounds. [Sheline & Pitzer J Am Chem Soc 72 1107 1950, Speyer & Wolf Chem Ber 60 1424 1927.] TOXIC.
Structure and conformation
Iron enneacarbonyl decomposes at 100°, but can be sublimed at 35°C in a high vacuum. It is insoluble in aliphatic hydrocarbons and reacts with many organic solvents to form mainly the pentacarbonyl or its substituted derivatives. These properties have considerably hindered structural studies ; the infrared spectrum, for example, can only be measured in the solid. The X-ray crystal structure determination shows it to have the structure shown in Fig. 8 in which six carbon monoxide molecules are arranged around each iron atom in a slightly distorted octahedral array. The Fe-Fe distance is 2.46A, whilst the Fe-C distances are all around 1.85A. The Mossbauer spectrum also suggests that the iron atoms are in an octahedral environment. However, since the compound is diamagnetic it must contain an Fe-Fe bond as well as the three bridging carbonyl groups.
Properties of DIIRON NONACARBONYL
| Melting point: | 100 °C |
| Boiling point: | 209°C (rough estimate) |
| Density | 2,85 g/cm3 |
| storage temp. | 2-8°C |
| form | crystal |
| Specific Gravity | 2.85 |
| color | yellow to orange |
| Water Solubility | Insoluble in water. |
| Sensitive | Air Sensitive |
| Exposure limits | ACGIH: TWA 1 mg/m3 NIOSH: TWA 1 mg/m3 |
| EPA Substance Registry System | Iron, tri-.mu.-carbonylhexacarbonyldi-, (Fe-Fe) (15321-51-4) |
Safety information for DIIRON NONACARBONYL
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H228:Flammable solids H261:Substances And Mixtures Which, In Contact With Water,Emit Flammable Gases |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P231+P232:Handle under inert gas. Protect from moisture. |
Computed Descriptors for DIIRON NONACARBONYL
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 DI IRON NONACARBONYL CAS 15321-51-4View Details
DI IRON NONACARBONYL CAS 15321-51-4View Details
15321-51-4 -
 Diironnonacarbonyl CAS 15321-51-4View Details
Diironnonacarbonyl CAS 15321-51-4View Details
15321-51-4 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1