Iron
Synonym(s):FE;Iron;TF2E1;TF2E2;TFIIE-A
- CAS NO.:7439-89-6
- Empirical Formula: Fe
- Molecular Weight: 55.85
- MDL number: MFCD00010999
- EINECS: 231-096-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
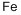
What is Iron?
Absorption
The efficiency of absorption depends on the salt form, the amount administered, the dosing regimen and the size of iron stores. Subjects with normal iron stores absorb 10% to 35% of an iron dose. Those who are iron deficient may absorb up to 95% of an iron dose.
Toxicity
Acute iron overdosage can be divided into four stages. In the first stage, which occurs up to six hours after ingestion, the principal symptoms are vomiting and diarrhea. Other symptoms include hypotension, tachycardia and CNS depression ranging from lethargy to coma. The second phase may occur at 6-24 hours after ingestion and is characterized by a temporary remission. In the third phase, gastrointestinal symptoms recur accompanied by shock, metabolic acidosis, coma, hepatic necrosis and jaundice, hypoglycemia, renal failure and pulmonary edema. The fourth phase may occur several weeks after ingestion and is characterized by gastrointestinal obstruction and liver damage. In a young child, 75 milligrams per kilogram is considered extremely dangerous. A dose of 30 milligrams per kilogram can lead to symptoms of toxicity. Estimates of a lethal dosage range from 180 milligrams per kilogram and upwards. A peak serum iron concentration of five micrograms or more per ml is associated with moderate to severe poisoning in many.
Description
Carbonyl iron is elemental iron produced by the decomposition of iron pentacarbonyl as a dark gray powder. When viewed under a microscope having a magnifying power of 500 diameters or greater, it appears as spheres built up with concentric shells. It is stable in dry air.
Description
Iron powder serves as a catalyst in a wide variety of reactions.
Chemical properties
Silver-white malleable metal. The only metal that can be tempered. Mechanical properties are altered by impurities, especially carbon.Iron is highly reactive chemically, a strong reducing agent, oxidizes readily in moist air, reacts with steam when hot, t
Chemical properties
Pure iron is a silvery-white, rather soft metal which is both malleable and ductile at room temperature. Its physical properties, however, are profoundly altered by the presence of trace amounts of other elements, and since pure iron finds little industrial use, the physical properties of the numerous steels are in many respects more important.
Physical properties
Pure iron is a silvery-white, hard, but malleable and ductile metal that can be worked andforged into many different shapes, such as rods, wires, sheets, ingots, pipes, framing, and soon. Pure iron is reactive and forms many compounds with other elements. It is an excellentreducing agent. It oxidizes (rusts) in water and moist air and is highly reactive with most acids,releasing hydrogen from the acid. One of its main properties is that it can be magnetized andretain a magnetic field.The iron with a valence of 2 is referred to as “ferrous” in compounds (e.g., ferrous chloride= FeCl2). When the valence is 3, it is called “ferric” (e.g., ferric chloride = FeCl3).Iron’s melting point is 1,535°C, its boiling point is 2,750°C, and its density is 7.873g/cm3.
Isotopes
There are 30 isotopes of iron ranging from Fe-45 to Fe-72. The following arethe four stable isotopes with the percentage of their contribution to the element’s naturalexistence on Earth: Fe-54 = 5.845%, Fe-56 = 91.72%, Fe-57 = 2.2%, and Fe-58 =0.28%. It might be noted that Fe-54 is radioactive but is considered stable because ithas such a long half-life (3.1×10+22 years). The other isotopes are radioactive and areproduced artificially. Their half-lives range from 150 nanoseconds to 1×105 years.
Origin of Name
The name “iron” or “iren” is Anglo-Saxon, and the symbol for iron (Fe) is from ferrum, the Latin word for iron.
Occurrence
Iron is the fourth most abundant element in the Earth s crust (about 5%) and is the ninth most abundant element found in the sun and stars in the universe. The core of the Earth is believed to consist of two layers, or spheres, of iron. The inner core is thought to be molten iron and nickel mixture, and the outer core is a transition phase of iron with the molten magma of the Earth s mantle. Iron s two oxide compounds (ferrous(II) oxide FeO) and (ferric(III) oxide Fe2O3) are the third and seventh most abundant compounds found in the Earth s crust. The most common ore of iron is hematite that appears as black sand on beaches or black seams when exposed in the ground. Small amounts of iron and iron alloys with nickel and cobalt were found in meteorites (siderite) by early humans. This limited supply was used to shape tools and crude weapons. Even though small amounts of iron compounds and alloys are found in nature (iron is not found in its pure metallic state in nature), early humans did not know how to extract iron from ores until well after they knew how to smelt gold, tin, and copper ores. From these metals, they then developed bronze alloy thus the Bronze Age (about 8000 BCE). There are several grades of iron ores, including hematite (brown ferric oxide) and limonite (red ferric oxide). Other ores are pyrites, chromite, magnetite, siderite, and low-grade taconite. Magnetite (Fe3O4) is the magnetic iron mineral/ore found in South Africa, Sweden, and parts of the United States. The lodestone, a form of magnetite, is a natural magnet. Iron ores are found in many countries. Iron is found throughout most of the universe, in most of the stars, and in our sun, and it probably exists on the other planets of our solar system.
History
Iron is a relatively abundant element in the universe. It is found in the sun and many types of stars in considerable quantity. It has been suggested that the iron we have here on Earth may have originated in a supernova. Iron is a very difficult element to produce in ordinary nuclear reactions, such as would take place in the sun. Iron is found native as a principal component of a class of iron–nickel meteorites known as siderites, and is a minor constituent of the other two classes of meteorites. The core of the Earth, 2150 miles in radius, is thought to be largely composed of iron with about 10% occluded hydrogen. The metal is the fourth most abundant element, by weight, making up the crust of the Earth. The most common ore is hematite (Fe2O3). Magnetite (Fe3O4) is frequently seen as black sands along beaches and banks of streams. Lodestone is another form of magnetite. Taconite is becoming increasingly important as a commercial ore. Iron is a vital constituent of plant and animal life, and appears in hemoglobin. The pure metal is not often encountered in commerce, but is usually alloyed with carbon or other metals. The pure metal is very reactive chemically, and rapidly corrodes, especially in moist air or at elevated temperatures. It has four allotropic forms,or ferrites, known as α, β, γ, and δ, with transition points at 700, 928, and 1530°C. The α form is magnetic, but when transformed into the β form, the magnetism disappears although the lattice remains unchanged. The relations of these forms are peculiar. Pig iron is an alloy containing about 3% carbon with varying amounts of S, Si, Mn, and P. It is hard, brittle, fairly fusible, and is used to produce other alloys, including steel. Wrought iron contains only a few tenths of a percent of carbon, is tough, malleable, less fusible, and usually has a “fibrous” structure. Carbon steel is an alloy of iron with carbon, with small amounts of Mn, S, P, and Si. Alloy steels are carbon steels with other additives such as nickel, chromium, vanadium, etc. Iron is the cheapest and most abundant, useful, and important of all metals. Natural iron contains four isotopes. Twenty-six other isotopes and isomers, all radioactive, are now recognized.
Characteristics
Iron is the only metal that can be tempered (hardened by heating, then quenching in wateror oil). Iron can become too hard and develop stresses and fractures. This can be corrected byannealing, a process that heats the iron again and then holds it at that temperature until thestresses are eliminated. Iron is a good conductor of electricity and heat. It is easily magnetized,but its magnetic properties are lost at high temperatures. Iron has four allotropic states. Thealpha form exists at room temperatures, while the other three allotropic forms exist at varyinghigher temperatures.Iron is the most important construction metal. It can be alloyed with many other metals tomake a great variety of specialty products. Its most important alloy is steel.An interesting characteristic of iron is that it is the heaviest element that can be formed byfusion of hydrogen in the sun and similar stars. Hydrogen nuclei can be “squeezed” in the sunto form all the elements with atomic numbers below cobalt (27Co), which includes iron. Itrequires the excess fusion energy of supernovas (exploding stars) to form elements with protonnumbers greater than iron (26Fe).
The Uses of Iron
Iron is a mineral used in food fortification that is necessary for the prevention of anemia, which reduces the hemoglobin concentra- tion and thus the amount of oxygen delivered to the tissues. sources include ferric ammonium sulfate, chloride, fructose, glycerophos- phate, nitrate, phosphate, pyrophosphate and ferrous ammonium sulfate, citrate, sulfate, and sodium iron edta. the ferric form (fe3+) is iron in the highest valence state and the ferrous form (fe2+) is iron in a lower valence state. the iron source should not discolor or add taste and should be stable. iron powders produce low discoloration and rancidity. it is used for fortification in flour, baked goods, pasta, and cereal products.
The Uses of Iron

To a solution of the SM (70 g, 386 mmol) in AcOH (100 mL) and absolute EtOH (400 mL) was slowly added iron powder (40 g). The reaction was cooled in an ice-H2O bath and treated with conc. HCl (1 mL). The addition was exothermic. The reaction was heated to reflux for 20 min, after which time it was allowed to cool to room temperature. The mixture was filtered and the filtrate concentrated to a thick oil. The resulting oil was partitioned between EtOAc (500 mL) and H2O (200 mL) and basified to pH 10 using 6N NaOH. The mixture was filtered through celite. The org layer was separated and washed with sat aq NaHCO3 (2 x 100 mL), H2O (2 x 100 mL), brine (100 mL), dried (Na2SO4), and concentrated to provide the product. [37.2 g, 64%]
The Uses of Iron
Smelting of iron from its ore occurs in a blast furnace where carbon (coke) and limestoneare heated with the ore that results in the iron in the ore being reduced and converted tomolten iron, called “pig iron.” Melted pig iron still contains some carbon and silicon as wellas some other impurities as it collects in the bottom of the furnace with molten slag floatingatop the iron. Both are tapped and drained off. This process can be continuous since moreingredients can be added as the iron and slag are removed from the bottom of the furnace.This form of iron is not very useful for manufacturing products, given that it is brittle andnot very strong.One of the major advances in the technology of iron smelting was the development of theBessemer process by Henry Bessemer (1813–1898). In this process, compressed air or oxygenis forced through molten pig iron to oxidize (burn out) the carbon and other impurities. Steelis then produced in a forced oxygen furnace, where carbon is dissolved in the iron at very hightemperatures. Variations of hardness and other characteristics of steel can be achieved with theaddition of alloys and by annealing, quench hardening, and tempering the steel.Powder metallurgy (sintering) is the process whereby powdered iron or other metals arecombined together at high pressure without high heat to fit molded forms. This process is usedto produce homogenous (uniform throughout) metal parts.One of the most useful characteristics of iron is its natural magnetism, which it loses athigh temperatures. Magnetism can also be introduced into iron products by electrical induction. Magnets of all sizes and shapes are used in motors, atom smashers, CT scanners, and TV and computer screens, toname a few uses. Super magnets can be formed by addingother elements (see cobalt) tohigh-quality iron.Iron is an important element making up hemoglobinin the blood, which carriesoxygen to the cells of ourbodies. It is also very important as a trace element inthe diet, assisting with theoxidation of foods to produce energy. We need about10 to 18 milligrams of ironeach day, as a trace mineral.Iron is found in liver andmeat products, eggs, shellfish, green leafy vegetables,peas, beans, and whole graincereals. Iron deficiency maycause anemia (low red bloodcell count), weakness, fatigue,headaches, and shortness ofbreath. Excess iron in thediet can cause liver damage,but this is a rare condition.
The Uses of Iron
Pure iron is very much a laboratory material and finds no great industrial use.
Indications
Used in preventing and treating iron-deficiency anemia.
Background
A metallic element found in certain minerals, in nearly all soils, and in mineral waters. It is an essential constituent of hemoglobin, cytochrome, and other components of respiratory enzyme systems. Its chief functions are in the transport of oxygen to tissue (hemoglobin) and in cellular oxidation mechanisms. Depletion of iron stores may result in iron-deficiency anemia. Iron is used to build up the blood in anemia.
What are the applications of Application
Iron is a common metal
Definition
Metallic element of atomic number 26, group VIII of the periodic table, aw 55.847, valences = 2,3; four stable isotopes, 4 artificially radioactive isotopes.
Definition
iron: Symbol Fe. A silvery malleableand ductile metallic transition element;a.n. 26; r.a.m. 55.847; r.d.7.87; m.p. 1535°C; b.p. 2750°C. Themain sources are the ores haematite(Fe2O3), magnetite (Fe3O4), limonite(FeO(OH)nH2O), ilmenite (FeTiO3),siderite (FeCO3), and pyrite (FeS2).The metal is smelted in a blast furnaceto give impure pig iron, whichis further processed to give castiron, wrought iron, and varioustypes of steel. The pure element hasthree crystal forms: alpha-iron, stablebelow 906°C with a body-centredcubicstructure; gamma-iron, stablebetween 906°C and 1403°C with anonmagnetic face-centred-cubicstructure; and delta-iron, which isthe body-centred-cubic form above1403°C. Alpha-iron is ferromagneticup to its Curie point (768°C). The elementhas nine isotopes (mass numbers52–60), and is the fourth mostabundant in the earth’s crust. It is requiredas a trace element (see essentialelement) by living organisms.Iron is quite reactive, being oxidizedby moist air, displacing hydrogenfrom dilute acids, and combiningwith nonmetallic elements. It formsionic salts and numerous complexeswith the metal in the +2 or +3 oxidationstates. Iron(VI) also exists in theferrate ion FeO42-, and the elementalso forms complexes in which its oxidationnumber is zero (e.g. Fe(CO)5).
Production Methods
Iron ore reserves are found worldwide. Areas with more than 1 billion metric tons of reserves include Australia, China, Brazil, Canada, the United States, Venezuela, South Africa, India, the former Soviet Union, Gabon, France, Spain, Sweden, and Algeria. The ore exists in varying grades, ranging from 20 to 70% iron content. North America has been fortunate in its ore deposits. There are commercially usable quantities in 22 U.S. states and in six Canadian provinces. In the United States the most abundant supplies, discovered in the early 1890s, are located in the Lake Superior region around the Mesabi Range. Other large deposits are found in Alabama, Utah, Texas, California, Pennsylvania, and New York. These deposits, particularly the Mesabi Range reserves, seemed inexhaustible in the 1930s when an average of 30 million tons of ore was produced annually from that one range. The tremendous demand for iron ore duringWorldWar II virtually tripled the output of the Mesabi Range and severely depleted its deposits of high-grade ore. The major domestic (U.S.) production is nowfrom crude iron ore, mainly taconite, a low-grade ore composed chiefly of hematite [FeO(OH) ·H2O] and silica found in the Great Lakes region.
Preparation
Most iron produced today is from its oxide minerals, hematite and magnetite. The process involves reducing mineral iron with carbon in a blast furnace. There are several types of blast furnaces which vary in design anddimensions. The overall processes, however, are more or less the same. Onesuch process is outlined below:
The mixture of ore, coke and limestone is fed into the blast furnace from thetop. The materials are preheated to about 200°C in the top most zone.Hematite is partially reduced to magnetite and then to FeO by the ascendingstream of carbon monoxide formed at the bottom and mid zones of the furnaceresulting from high temperature oxidation of carbon. The ferrous oxide FeOformed at the top zone is reduced to metallic iron at about 700°C in the midzone by carbon monoxide. A hot air blast at 900°C passes through the entirefurnace for a very short time (usually for a few seconds). This prevents any gassolid reaction product from reaching equilibrium. In the temperature zone 700to 1,200°C ferrous oxide is completely reduced to iron metal by carbon monox-ide. Also, more CO is formed by oxidation of carbon by carbon dioxide. Furtherdown the furnace at higher temperatures, around 1,500°C, iron melts, drippingdown into the bottom. Also, in this temperature zone acidic silica particlesreact with basic calcium oxide produced from the decomposition of limestone,producing calcium silicate. The molten waste calcium silicate also drips downinto the bottom. In the hottest zone of the blast furnace, between 1,500 to2,000°C, some carbon dissolves into the molten iron. Also at these temperatures any remaining silicates and phosphates are reduced to silicon and phosphorus, and dissolve into the molten iron. Additionally, other tract metals suchas manganese dissolve into the molten iron. The impure iron melt containingabout 3 to 4% carbon is called “pig iron”. At the bottom, the molten waste slagfloats over the impure pig iron melt that is heavier than the slag melt andimmiscible with it. Pig iron is separated from the slag and purified for makingdifferent types of steel. Chemical reactions and processes occurring in varioustemperature zones of blast furnace are summarized below:
Pig iron produced in the blast furnace is purified and converted to steel ina separate furnace, known as a basic-oxygen furnace. Jets of pure oxygen gasat high pressure are blown over and through the pig iron melt. Metal impurities are converted into oxides. Part of the dissolved carbon in the impure ironmelt is converted into carbon dioxide gas. Formation of SiO2, CO2,and othermetal oxides are exothermic reactions that raise the temperature to sustainthe melt. A lime flux (CaO) also is added into the melt, which converts silicainto calcium silicate, CaSiO3,and phosphorus into calcium phosphate,Ca3(PO4)2,forming a molten slag immiscible with molten steel. The lightermolten slag is decanted from the heavier molten steel.
General Description
A gray lustrous powder. Used in powder metallurgy and as a catalyst in chemical manufacture.
Air & Water Reactions
Highly flammable. May react with water to give off hydrogen, a flammable gas. The heat from this reaction may ignite the hydrogen.
Reactivity Profile
Iron is pyrophoric [Bretherick, 1979 p. 170-1]. A strong reducing agent and therefore incompatible with oxidizing agents. Burns in chlorine gas [Mellor 2, Supp. 1:380 1956]. Reacts with fluorine with incandescence [Mellor 13:314, 315, 1946-1947].
Hazard
Iron dust from most iron compounds is harmful if inhaled and toxic if ingested. Iron dustand powder (even filings) are flammable and can explode if exposed to an open flame. Asmentioned, excessive iron in the diet may cause liver damage.
Health Hazard
Fire may produce irritating and/or toxic gases. Contact may cause burns to skin and eyes. Contact with molten substance may cause severe burns to skin and eyes. Runoff from fire control may cause pollution.
Fire Hazard
Flammable/combustible material. May be ignited by friction, heat, sparks or flames. Some may burn rapidly with flare burning effect. Powders, dusts, shavings, borings, turnings or cuttings may explode or burn with explosive violence. Substance may be transported in a molten form at a temperature that may be above its flash point. May re-ignite after fire is extinguished.
Biochem/physiol Actions
Carbonyl iron has a role as an absorber of microwave radiation. Carbonyl iron also exhibits shielding properties because of the low density and the connectivity among the fillers it provide. It is used in industries and in the synthesis of nitro-group-containing pharmaceutical ingredients.
Pharmacokinetics
The major activity of supplemental iron is in the prevention and treatment of iron deficiency anemia. Iron has putative immune-enhancing, anticarcinogenic and cognition-enhancing activities.
Environmental Fate
Iron occurs rarely by itself in nature due to the ease with which it forms compounds, especially in oxidation reactions. Many iron compounds are water soluble, leading to potentially high concentrations in water, especially in seawater. Iron is a necessary component of all life and it is therefore taken up readily by organisms from all sources.
Metabolism
Not Available
Purification Methods
Clean it in conc HCl, rinse in de-ionised water, then reagent grade acetone and dry it under vacuum.
Structure and conformation
Two structural types of iron occur in the solid state. At room temperature iron has a body-centered cubic lattice (the a form). At about 910°C the a form is transformed into the γ allotrope which has a cubic close-packed structure. Around 1390°C a body-centred cubic lattice is reformed—the δ form. Thus the allotropy of iron is unusual in that it can exist with the same crystal form in two distinct temperature ranges which are separated by a range within which a different form is stable. The a and ? forms have similar lattice parameters— the differences between them being expected in view of thermal expansion which increases the size of the unit cell of the δallotrope.
Toxicity evaluation
In some adults, iron overload can be the result of a genetic
defect (idiopathic hemochromatosis) that causes malfunction of the normal homeostasis mechanism and, in turn, excessive
absorption of iron. Iron overload can also be caused by too
many blood transfusions, which results in too much iron in the
various iron-containing organs.
Recently, it has been suggested that the presence of
increased transferrin concentrations in males is associated with
an increased number of heart attacks. This must be corroborated
by further research. Excess iron can lead to diabetes
mellitus, faulty liver functions, and endocrine disturbance.
Iron is a catalyst for oxidative damage leading to lipid peroxidation.
The latest hypotheses link peroxidation to heart
disease, cancer, and accelerated aging. Iron is involved in the
Fenton reaction, which catalyzes the formation of free radicals
that cause excessive damage to cells and their components.
Properties of Iron
| Melting point: | 1535 °C(lit.) |
| Boiling point: | 2750 °C(lit.) |
| Density | 7.86 g/mL at 25 °C(lit.) |
| Flash point: | >230 °F |
| storage temp. | 2-8°C |
| solubility | H2O: soluble |
| form | wire |
| appearance | Gray powder |
| color | Silvery |
| Specific Gravity | 7.86 |
| Odor | Odorless |
| Resistivity | 9.71 μΩ-cm |
| Water Solubility | INSOLUBLE |
| Sensitive | Moisture Sensitive |
| Merck | 13,5109 |
| Exposure limits | ACGIH: TWA 2 mg/m3 OSHA: TWA 15 mg/m3; TWA 5 mg/m3 NIOSH: IDLH 1250 mg/m3; TWA 2.5 mg/m3 |
| Stability: | Stable. Reacts slowly with moist air and water. Dust may form an explosive or combustible mixture with air. Incompatible with organic acids, strong oxidizing agents, water, mineral acids. |
| CAS DataBase Reference | 7439-89-6(CAS DataBase Reference) |
| NIST Chemistry Reference | Iron(7439-89-6) |
| EPA Substance Registry System | Iron (7439-89-6) |
Safety information for Iron
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02 |
| GHS Hazard Statements |
H228:Flammable solids |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. |
Computed Descriptors for Iron
Iron manufacturer
INDUSTRIAL METAL POWDERS (INDIA) PVT LTD
ARRAKIS INDUSTRIES LLP
New Products
Methyl (R)-1-Boc-4,4-difluoropyrrolidine-2-carboxylate 2,2-Difluoropropylamine hydrochloride tert-butyl 3-bromoazetidine-1-carboxylate (R)-1-Boc-3-hydroxypyrrolidine DIFLUOROACETIC ANHYDRIDE 2,2-Difluoropropionic acid Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) Dibenzoyl Peroxide Titanium Dioxide 2-(Methylthio) Benzonitrile Sodium Acetate Anhydrous Allopurinol 1,5-DibromopentaneRelated products of tetrahydrofuran






You may like
-
 Stainless Steel foil CASView Details
Stainless Steel foil CASView Details -
 Stainless Steel foil CASView Details
Stainless Steel foil CASView Details -
 Stainless Steel foil CASView Details
Stainless Steel foil CASView Details -
 Stainless Steel foil CASView Details
Stainless Steel foil CASView Details -
 Stainless Steel foil CASView Details
Stainless Steel foil CASView Details -
 Stainless Steel foil CASView Details
Stainless Steel foil CASView Details -
 Stainless Steel foil CASView Details
Stainless Steel foil CASView Details -
 Stainless Steel foil CASView Details
Stainless Steel foil CASView Details
