Ironpentacarbonyl
- CAS NO.:13463-40-6
- Empirical Formula: 5CO.Fe
- Molecular Weight: 195.9
- MDL number: MFCD00011001
- EINECS: 236-670-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:32
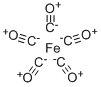
What is Ironpentacarbonyl?
Chemical properties
yellow-orange to brown liquid which
The Uses of Ironpentacarbonyl
To make finely divided iron, so-called carbonyl iron, which is used in the manufacture of powdered iron cores for high frequency coils used in the radio and television industry; as antiknock agent in motor fuels; as catalyst and reagent in organic reactions.
The Uses of Ironpentacarbonyl
It is used to produce carbonyl iron (finelydivided iron) for high frequency coils andalso for radio and television (Merck 1996).It is also used as a catalyst in many organicsyntheses and as an antiknock agent in motorfuels.
The Uses of Ironpentacarbonyl
As strong reducing agent; in manufacture of high-frequency coils used in radios and televisions; as antiknock agent in motor fuels
General Description
A yellow to dark red liquid. Insoluble in water and denser than water. Very toxic by inhalation, ingestion, and skin absorption. Flash point 5°F. Used to make other chemicals.
Air & Water Reactions
Highly flammable. Ironpentacarbonyl is spontaneously flammable in air, [R. Kamo, IIT Progs. Rept. 1, p. 23(1962)]. Insoluble in water.
Reactivity Profile
Organometallics, such as Ironpentacarbonyl, are reactive with many other groups. Incompatible with acids and bases. Organometallics are good reducing agents and therefore incompatible with oxidizing agents. Often reactive with water to generate toxic or flammable gases. A brown pyrophoric powder is produced by the combination of the carbonyl with acetic acid containing greater than 5% of water.
Health Hazard
Toxicity of Ironpentacarbonyl is high via all routes of entry. Cyanosis (bluish discoloration of skin) and circulatory collapse may occur after exposure. Death may result. Pneumonitis and injury to the kidneys, liver, and central nervous system may also occur.
Health Hazard
Iron pentacarbonyl is highly toxic, the acutetoxicity, however, is lower than that of nickeltetracarbonyl. The toxic symptoms, however,are nearly the same. Being highly volatile[the vapor pressure being 30 torr at 20°C(68°F)], this compound presents a seriousrisk of inhalation to its vapors. Furthermore,it evolves toxic carbon monoxide whenexposed to light. The reaction is as follows:
2Fe(CO)5→Fe2(CO)9 +CO
Therefore, all handling and operations mustbe carried out in fume hoods or under adequateventilation. Inhalation of its vapor can cause headache, dizziness, and somnolence.Other symptoms are fever, coughing andcyanosis, which may manifest several hoursafter exposure. The vapor is an irritant tothe lung. Chronic exposure may cause injuryto liver and kidneys. The inhalation LC50value in rats is 10 ppm for a 4-hour exposureperiod (RTECS 2004). The oral LD50value in rats is 25 mg/kg and in rabbits 12mg/kg. Any cancer-causing effect in animalsor humans has not been reported. Sodium saltof EDTA or dithiocarb salts of sodium or calciumare antidotes against iron pentacarbonylpoisoning.
Fire Hazard
Ironpentacarbonyl may be ignited by heat, sparks, or flames. Vapors may travel to ignition source and flash back. Containers may explode in the heat of fire. Evolution of carbon monoxide may create a poison hazard. Ironpentacarbonyl presents a vapor explosion and poison hazard indoors, outdoors, or in sewers. Evolves carbon monoxide on exposure to air or to light. Emits carbon monoxide when heated to decomposition. Avoid acetic acid, water, nitrogen oxide, transition metal halides, and zinc and Ironpentacarbonyl burns in air. Decomposes in acids and alkalies. Protect from light and air.
Purification Methods
It is a pale yellow viscous liquid which is PYROPHORIC and readily absorbed by the skin. HIGHLY TOXIC (protect from light and air). It should be purified in a vacuum line by distilling and collecting in a trap at -96o (toluene-Dry ice slush). It has been distilled at atmospheric pressure (use a very efficient fume cupboard). At 180o/atmospheric pressure it decomposes to give Fe and CO. In UV light in pet ether it forms Fe2(CO)9 (see previous entry). [Hagen et al. Inorg Chem 17 1369 1978, Ewens et al. Trans Faraday Soc 35 6811 1939.]
Properties of Ironpentacarbonyl
| Melting point: | -20 °C |
| Boiling point: | 103 °C(lit.) |
| Density | 1.49 g/mL at 25 °C(lit.) |
| vapor density | 6.74 (vs air) |
| vapor pressure | 35 mm Hg ( 25 °C) |
| refractive index | n |
| Flash point: | 5 °F |
| storage temp. | 2-8°C |
| solubility | insoluble in H2O; soluble in ethyl ether, benzene, acetone |
| form | Liquid Which May Contain Particulate Matter |
| color | Yellow-orange to brown |
| Specific Gravity | 1.490 |
| Water Solubility | Soluble in nickel tetracarbonyl and organic solvents. Insoluble in water |
| Sensitive | Air Sensitive |
| Hydrolytic Sensitivity | 3: reacts with aqueous base |
| Merck | 14,5099 |
| Exposure limits | TLV-TWA: 0.1 ppm (~0.8 mg(Fe)/m3) (ACGIH), 0.01 ppm (MSHA) TLV-STEL: 0.2 ppm (~1.6 mg(Fe)/m3) (ACGIH). |
| Stability: | Moisture Sensitive |
| CAS DataBase Reference | 13463-40-6(CAS DataBase Reference) |
| EPA Substance Registry System | Iron pentacarbonyl (13463-40-6) |
Safety information for Ironpentacarbonyl
| Pictogram(s) |
 Flame Flammables GHS02  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H225:Flammable liquids H300:Acute toxicity,oral H310:Acute toxicity,dermal H330:Acute toxicity,inhalation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P310:Immediately call a POISON CENTER or doctor/physician. P302+P352:IF ON SKIN: wash with plenty of soap and water. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. |
Computed Descriptors for Ironpentacarbonyl
Ironpentacarbonyl manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

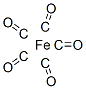
You may like
-
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 1-methylindoline-2,3-dione 98%View Details
1-methylindoline-2,3-dione 98%View Details
2058-74-4 -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
