Dihydrostreptomycin
- CAS NO.:128-46-1
- Empirical Formula: C21H41N7O12
- Molecular Weight: 583.59
- MDL number: MFCD00084778
- EINECS: 204-888-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-03-16 15:39:10
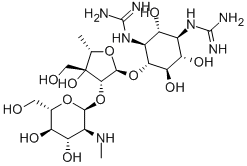
What is Dihydrostreptomycin?
Description
Dihydrostreptomycin was first synthesized by Parke Davis Co. in 1946by the reduction of streptomycin. Naturally occurring dihydrostreptomycin was found in the culture broth of Streptomyces humidus by Takeda Chemicals Industries in 1957. Its hydrochloride or sulfate is more easily crystallized and more stable under alkaline conditions than streptomycin. Dihydrostreptomycin has been used for therapy of tuberculosis, but because it has a higher ototoxicity than streptomycin its use is now restricted to animal therapy.
Originator
Dihydrostrepto,MSD ,US,1948
The Uses of Dihydrostreptomycin
Antibacterial.
Background
Dihydrostreptomycin is an aminoglycoside antibiotic. In humans, the use of dihydrostreptomycin has been associated with ototoxicity. The FDA withdrew its approval for the use of all drug products containing dihydrostreptomycin sulfate.
Definition
ChEBI: Dihydrostreptomycin is a member of streptomycins.
Manufacturing Process
Dihydrostreptomycin sulfate may be prepared from streptomycin sulfate by catalytic hydrogenation (Merck, Pfizer, Cyanamid), electrolytic reduction (Schenley, Olin Mathieson), or by sodium borohydride reduction (Bristol), or by isolation from a fermentation process (Takeda).
brand name
Abocillin;Biostrep;Complexobiotico;Diapenin 3;Diapenin balsamico;Diarrestival;Didromycin;Didrothenate;Dihydrocidan sulfato;Dihydrostreptofar;Diidro-pantostrept;Distreptopab;Dreiciclina balsamica;Dst;Entera-strept;Estreptoluy;Estreptosirup;Helle-strep-forte;Hp 48;Mastigun;Mixtencillin;Retromyopen;Rocopenstrep;Sanstrepto;Solmycin;Solvo-strept;Streptoduocin;Veticar;Veycil-as.
Therapeutic Function
Antibiotic
World Health Organization (WHO)
Dihydrostreptomycin, a derivative of the aminoglycoside antibiotic streptomycin with similar antibacterial activity, was first synthesized in 1947 and subsequently used in the treatment of tuberculosis and gram-negative infections. Preparations for systemic use have been widely withdrawn as a result of concern regarding their severe ototoxicity. Dihydrostreptomycin is poorly absorbed from the gastrointestinal tract. It remains available in oral preparations in some countries.
Safety Profile
Poison by intravenous and intramuscular routes. Moderately toxic by subcutaneous and intraperitoneal routes. Human teratogenic effects by unspecified route: developmental abnormahties of the eye and ear. An experimental teratogen. Other experimental reproductive effects. Mutation data reported. A derivative of streptomycin; has anesthetic properties. When heated to decomposition it emits toxic fumes of NOx
Metabolism
Not Available
Properties of Dihydrostreptomycin
| Melting point: | >300 °C |
| Boiling point: | 641.09°C (rough estimate) |
| Density | 1.3963 (rough estimate) |
| refractive index | 1.6800 (estimate) |
| pka | pKa 7.8 (Uncertain) |
Safety information for Dihydrostreptomycin
Computed Descriptors for Dihydrostreptomycin
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-(2,4-Dimethoxybenzyl)dihydropyrimidine-2,4(1H,3H)-dione 7-Bromo-1H-indazole N-octanoyl benzotriazole 3,4-Dibenzyloxybenzaldehyde 4-Hydrazinobenzoic acid Electrolytic Iron Powder Fmoc-Val-Cit-PAB 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 4-HYDROXY BENZYL ALCOHOL 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) S-2-CHLORO PROPIONIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 1-(4-Methylphenylsulfonyl)-1H-1,2,3-benzotriazole 1-(2-Chlorobenzyl)-4-nitro-1H-pyrazole 1-(2-Nitrophenyl)-4-phenylpiperazineRelated products of tetrahydrofuran



You may like
-
 55441-95-7 2 2-BIS(2-HYDROXYETHOXY)-1 1-BINAPHTHYL 99%View Details
55441-95-7 2 2-BIS(2-HYDROXYETHOXY)-1 1-BINAPHTHYL 99%View Details
55441-95-7 -
 181228-33-1 99%View Details
181228-33-1 99%View Details
181228-33-1 -
 Ste-Glu-AEEA-AEEA-OSUView Details
Ste-Glu-AEEA-AEEA-OSUView Details
1169630-40-3 -
 1446013-08-6 Fmoc-His-Aib-OH TFA 98%View Details
1446013-08-6 Fmoc-His-Aib-OH TFA 98%View Details
1446013-08-6 -
 127464-43-1 99%View Details
127464-43-1 99%View Details
127464-43-1 -
 Chloro Uracil 99%View Details
Chloro Uracil 99%View Details
1820-81-1 -
 2-ETHYLPYRIDINE 100-71-0 99%View Details
2-ETHYLPYRIDINE 100-71-0 99%View Details
100-71-0 -
 13162-05-5 99%View Details
13162-05-5 99%View Details
13162-05-5