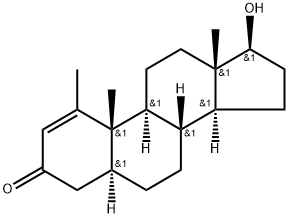
Stanolone
Synonym(s):DHT;Dihydrotestosterone;Stanolone;Androstanolone;17β-Hydroxy-5α-androstan-3-one
- CAS NO.:521-18-6
- Empirical Formula: C19H30O2
- Molecular Weight: 290.45
- MDL number: MFCD00003667
- EINECS: 208-307-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:32
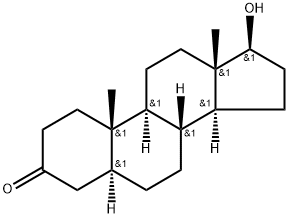
What is Stanolone?
Description
Stanolone is well known as dihydrotestosterone (DHT), which is an endogenous androgen sex steroid and hormone. It is an agonist of the androgen receptor (AR). It plays important physiological role in sexual differentiation, maturation of the penis and scrotum, hair, sebum production and development and maintenance of the prostate gland and seminal vesicles. It is mainly used for the treatment of male hypogonadism, androgen deficiency of severe illness, androgen deficiency of ageing and microphallus in infancy.
Chemical properties
White Crystalline Solid
Originator
Neodrol,Pfizer,US,1953
The Uses of Stanolone
Stanolone is a potent androgenic metabolite of testosterone that It is Controlled substance. It was created for the treatment of muscle wasting disease, and osteoporosis. It is a powerful anabolic steroid, which is similar to the body's naturally produced Dihydrotestosterone. Dihydrotestosterone (DHT) is generated by a 5-alpha reduction of testosterone. Unlike testosterone, DHT cannot be aromatized to estradiol therefore DHT is considered a pure androgenic steroid.
Definition
ChEBI: Stanolone is a 17beta-hydroxy steroid that is testosterone in which the 4,5 double bond has been reduced to a single bond with alpha-configuration at position 5. It has a role as an androgen, a human metabolite, a Daphnia magna metabolite and a mouse metabolite. It is a 17beta-hydroxy steroid, a 17beta-hydroxyandrostan-3-one and a 3-oxo-5alpha-steroid. It derives from a hydride of a 5alpha-androstane.
Manufacturing Process
A solution of 1.0 g of 3,17-androstandione in 50 ml of methanol and containing 1 g of selenium dioxide, was allowed to remain in an ice-chest overnight. The formed 3,3-dimethoxyandrostan-17-one was not separated. 1 g of solid potassium hydroxide and 2.5 g of sodium borohydride in 2.5 ml of water were added and the mixture allowed to react at room temperature for 24 hours. The solution was then poured into a large excess of water, extracted with methylene chloride, the organic layer dried and evaporated to a residue. The residue was dissolved in ether, and a small amount of selenium removed by filtration. The ether was boiled off and the organic material dissolved in 100 ml of boiling acetone. 25 ml of diluted hydrochloric acid were added, the solution boiled for 5 minutes and then allowed to cool. Upon crystallization, 0.85 g of androstan-17β-ol-3-one was obtained, melting point 175°C to 178°C.
Therapeutic Function
Androgen
General Description
Dihydrotestosterone (Item No. 15874) is an analytical reference standard categorized as an anabolic androgenic steroid. Anabolic steroids, including dihydrotestosterone, have been used to enhance physical performance in athletes. Dihydrotestosterone is regulated as a Schedule III compound in the United States. This product is intended for research and forensic applications.
Side Effects
In rare cases, serious and even fatal cases of liver problems have developed during treatment with stanozolol. Contact your doctor immediately if you experience abdominal pain, light-colored stools, dark-colored urine, unusual fatigue, nausea or vomiting, or yellowing of the skin or eyes. These may be early signs of liver problems.
Serious side effects: an allergic reaction (difficulty breathing; closing of the throat; swelling of the lips, tongue, or face; or hives);
swelling of the arms or legs (especially ankles);
frequent or persistent erections, or breast tenderness or enlargement (male patients); or
voice changes (hoarseness, deepening), hair loss, facial hair growth, clitoral enlargement, or menstrual irregularities (female patients).
Common stanozolol side effects may include:
new or worsening acne;
difficulty sleeping;
headache; or
changes in sexual desire.
References
https://en.wikipedia.org/wiki/Dihydrotestosterone#Medical_use
Swerdloff, R. S., and C. Wang. "Dihydrotestosterone: a rationale for its use as a non-aromatizable androgen replacement therapeutic agent."Baillieres Clin Endocrinol Metab 12.3(1998):501.
Properties of Stanolone
| Melting point: | 178-183 °C |
| Boiling point: | 372.52°C (rough estimate) |
| alpha | 27 º |
| Density | 1.0320 (rough estimate) |
| refractive index | 1.4709 (estimate) |
| Flash point: | 9℃ |
| storage temp. | 2-8°C |
| solubility | Acetonitrile: 1 mg/ml; Ethanol: 1 mg/ml; Methanol: 1 mg/ml |
| pka | 15.08±0.60(Predicted) |
| form | neat |
| Water Solubility | 344.3g/L(temperature not stated) |
| Merck | 13,8872 |
| BRN | 2056371 |
| CAS DataBase Reference | 521-18-6(CAS DataBase Reference) |
| NIST Chemistry Reference | Stanolone(521-18-6) |
| EPA Substance Registry System | Androstan-3-one, 17-hydroxy-, (5.alpha.,17.beta.)- (521-18-6) |
Safety information for Stanolone
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H351:Carcinogenicity H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P273:Avoid release to the environment. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Stanolone
| InChIKey | NVKAWKQGWWIWPM-ABEVXSGRSA-N |
Stanolone manufacturer
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran




You may like
-
 521-18-6 Testosterone EP Impurity F 98%View Details
521-18-6 Testosterone EP Impurity F 98%View Details
521-18-6 -
 5α-Dihydrotestosterone (DHT) solution CAS 521-18-6View Details
5α-Dihydrotestosterone (DHT) solution CAS 521-18-6View Details
521-18-6 -
 5α-Androstan-17β-ol-3-one CAS 521-18-6View Details
5α-Androstan-17β-ol-3-one CAS 521-18-6View Details
521-18-6 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8